Abstract
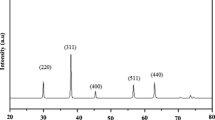
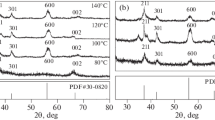
MnO2 exhibits numerous catalytic applications owing to its wide structural diversity with different chemical and physical properties. Different morphologies of MnO2 nanomaterial have been prepared to investigate oxidation of methylene blue dye and reduction of nitrobenzene under natural sunlight. MnO2 nanostructures displayed versatile photoactivity due to different surface and crystal structural morphologies. The prepared materials were characterized by XRD, SEM, EDS, DRS, DLS and fluorescence spectroscopy. The change in absorption maxima from 441 (sea urchin) to 444 nm (nanospheres) is due to change in shape, size and exposure of surface ligand density. Particle size distribution of α-MnO2 nanorods, sea urchin is 51 and 90–190 nm, respectively, with body-centred tetragonal crystalline structure. The structure of synthesized polymorphs consists of octahedral (MnO6) with different arrangements of edge and phase sharing. From SEM, variation in shapes from sea urchin to nanorods to nanospheres can be seen. MnO2-sea urchin (98%) and α-MnO2 nanorods (91%) give better activity in photo-oxidation of dye relative to MnO2 nanospheres. The overall photo-oxidation reaction follows pseudo first-order kinetics. Nitrobenzene (25 μmol) is selectively reduced to 80% aniline (20.5 μmol) by α-nanotubes of MnO2 catalyst and 82.2% with MnO2-sea urchin photocatalyst. This paper explored the correlations between shape-dependent chemical and structural factors of surface of a catalyst. The excellent performance of hierarchical MnO2 represents a potential heterogeneous catalyst for wastewater treatment.








Similar content being viewed by others
References
Wang Y C, Kan B, Yu Y, Pan K M, Liew L and Song Y 2017 Composites: Part A 95 173
Crespo Y and Seriani N 2014 J. Mater. Chem. A 2 16538
Cao J, Mao Q, Shi L and Qian Y 2011 J. Mater. Chem. 21 16210
Wang X and Li Y D J 2002 J. Am. Chem. Soc. 124 2880
Wang X and Li Y 2003 Chem. Eur. J. 9 300
Subramanian V, Zhu H W, Vajtai R, Ajayan P M and Wei B Q 2005 J. Phys. Chem. B 109 20207
Albering J H 1999 Handbook of battery materials. Part II: materials for aqueous electrolyte batteries, structural chemistry of manganese dioxide and related compounds (New York: McGraw-Hill)
Thackeray M M 1997 Prog. Solid State Chem. 25 1
Chabre Y and Pannetier J 1995 Prog. Solid State Chem. 23 1
Shen Y F, Zerger R P, DeGuzman R N, Suib S L, McCurdy L, Potter D I et al 1993 Science 260 511
Duay J, Sherrill S A, Gui Z, Gillette E and Lee S B 2013 ACS Nano 7 1200
Teng F, Santhanagopalan S and Meng D D 2010 Solid State Sci. 12 1677
Kaur J and Pal B 2014 Catal. Commun. 53 25
Rather R, Singh S and Pal B 2016 J. Ind. Eng. Chem. 37 288
Kaur R and Pal B 2015 Appl. Catal. A: General 491 28
Greenwood Norman N and Alan E 1985 Chemistry of the elements (Oxford: Pergamon Press)
Feng Q, Kanoh H and Ooi K 1999 J. Mater. Chem. 9 319
Zhang Y X, Guo X L, Huang M, Hao X D, Yuan Y and Hua C 2015 J. Phys. Chem. Solids 83 40
Zeng J, Nair J R, Francia C, Bodoardo S and Penazzi N 2013 Int. J. Electrochem. Sci. 8 3912
Débart A, Paterson A J, Bao J and Bruce P G 2008 Angew. Chem. 120 4597
Meng Y, Song W, Huang H, Ren Z, Chen S Y and Suib S L 2014 J. Am. Chem. Soc. 136 11452
Park M S, Kim J H, Kim K J, Jeong G and Kim Y J 2013 J. Nanosci. Nanotech. 13 3611
Li Y, Wan Y, Zhan S, Guan Q and Tian Y 2016 RSC Adv. 6 54926
Wang X and Li Y D J 2002 Am. Chem. Soc. 124 2880
Yang X, Tang T, Feng Q and Ooi K 2003 Cryst. Growth Des. 3 409
Selvakumar K, Senthil Kumar S M, Thangamuthu R, Ganesan K P, Murugan P, Rajput P et al 2015 J. Phys. Chem. C 119 6604
Chen H and He J 2008 J. Phys. Chem. C 112 17540
Pike J, Hanson J, Zhang L and Chan S W 2007 Chem. Mater. 19 5609
Barai H R, Banerjee A R, Hamnabard N and Joo S W 2016 RSC Adv. 6 78887
Aguilar-Tapia A, Delannoy L, Louis C, Han C W, Ortalan V and Zanella R 2016 J. Catal. 344 515
Acknowledgement
We are grateful to the Department of Science and Technology (DST), Government of India, for providing financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thakur, S., Tinna, D. & Pal, B. Surface structure, morphology and crystal phase-dependent photoactivity of MnO2 nanocatalysts under sunlight. Bull Mater Sci 44, 231 (2021). https://doi.org/10.1007/s12034-021-02520-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-021-02520-4