Abstract—
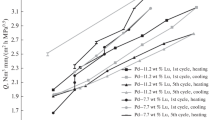
We have studied the effect of surface lamp processing on the hydrogen permeability of membrane foil produced by rolling an ingot of a Pd–Cu solid solution. Such processing has been shown to remove sorption products from the membrane surface and to significantly improve the hydrogen permeability of the membranes at temperatures of up to 300°C. The use of a reactor with a cleaned PdCu membrane allows the hydrogen yield in the methanol steam reforming process in the presence of a Ni0.2Cu0.8/Ce0.3Zr0.7O2–δ catalyst to be raised relative to that in a conventional flow reactor owing to the displacement of thermodynamic equilibrium as a result of the removal of hydrogen from the reaction zone. The effect of membrane surface cleaning by lamp processing is most clearly demonstrated by examining the yield of high-purity hydrogen in the permeate zone. Whereas at 360°C the yield of high-purity hydrogen in a reactor containing a membrane with a cleaned surface increased by just 15%, membrane surface cleaning by lamp processing ensured a 15‑fold gain at 260°C. This is due to the considerable difference between the hydrogen permeabilities of the membranes at low temperatures and its gradual decrease at high temperatures.









Similar content being viewed by others
REFERENCES
Widén, J., Carpman, N., Castellucci, V., Lingfors, D., Olauson, J., Remouit, F., Bergkvist, M., Grabbe, M., and Waters, R., Variability assessment and forecasting of renewables: a review for solar, wind, wave and tidal resources, Renewable Sustainable Energy Rev., 2015, vol. 44, pp. 356–375. https://doi.org/10.1016/j.rser.2014.12.019
Yaroslavtsev, A.B., Stenina, I.A., and Golubenko, D.V., Membrane materials for energy production and storage, Pure Appl. Chem., 2020, vol. 92, no. 7, pp. 1147–1157. https://doi.org/10.1515/pac-2019-1208
Kaur, M. and Pal, K., Review on hydrogen storage materials and methods from an electrochemical viewpoint, J. Energy Storage, 2019, vol. 23, pp. 234–249. https://doi.org/10.1016/j.est.2019.03.020
Tsodikov, M.V., Arapova, O.V., Konstantinov, G.I., Bukhtenko, O.V., Ellert, O.G., Nikolaev, S.A., and Vasil’kov, A.Y., The role of nanosized nickel particles in microwave-assisted dry reforming of lignin, Chem. Eng. J., 2017, vol. 309, pp. 628–637. https://doi.org/10.1016/j.cej.2016.10.031
Lytkina, A.A., Orekhova, N.V., and Yaroslavtsev, A.B., Catalysts for the steam reforming and electrochemical oxidation of methanol, Inorg. Mater., 2018, vol. 54, no. 13, pp. 1315–1329. https://doi.org/10.1134/S0020168518130034
World Energy Outlook 2019, Paris: Int. Energy Agency, 2019.
Tsodikov, M.V., Kurdymov, S.S., Konstantinov, G.I., Murzin, V.Y., Bukhtenko, O.V., and Maksimov, Y.V., Core–shell bifunctional catalyst for stream methane reforming resistant to H2S: activity and structure evolution, Int. J. Hydrogen Energy, 2015, vol. 40, pp. 2963–2970. https://doi.org/10.1016/j.ijhydene.2015.01.016
Sánchez-Bastardo, N., Schlögl, R., and Ruland, H., Methane pyrolysis for CO2-free H2 production: a green process to overcome renewable energies unsteadiness, Chem. Eng. Tech., 2020, vol. 92, pp. 1596–1609. https://doi.org/10.1002/cite.202000029
Torres, D., Pinilla, J.L., and Suelves, I., Screening of Ni–Cu bimetallic catalysts for hydrogen and carbon nanofilaments production via catalytic decomposition of methane, Appl. Catal., A, 2018, vol. 559, pp. 10–19. https://doi.org/10.1016/j.apcata.2018.04.011
Ouyang, M.Z., Boldrin, P., Maher, R.C., Chen, X.L., Liu, X.H., Cohen, L.F., and Brandon, N.P., A mechanistic study of the interactions between methane and nickel supported on doped ceria, Appl. Catal., B, 2019, vol. 248, pp. 332–340. https://doi.org/10.1016/j.apcatb.2019.02.038
Pudukudy, M., Yaakob, Z., Jia, Q., and Takriff, M.S., Catalytic decomposition of methane over rare earth metal (Ce and La) oxides supported iron catalysts, Appl. Surf. Sci., 2019, vols. 467–468, pp. 236–248. https://doi.org/10.1016/j.apsusc.2018.10.122
Nishii, H., Miyamoto, D., Umeda, Y., Hamaguchi, H., Suzuki, M., Tanimoto, T., Harigai, T., Takikawa, H., and Suda, Y., Catalytic activity of several carbons with different structures for methane decomposition and by-produced carbons, Appl. Surf. Sci., 2019, vol. 473, pp. 291–297. https://doi.org/10.1016/j.apsusc.2018.12.073
Pinaeva, L.G., Noskov, A.S., and Parmon, V.N., Prospects for the direct catalytic conversion of methane into useful chemical products, Catal. Ind., 2017, vol. 9, pp. 283–298. https://doi.org/10.1134/S2070050417040067
He, J., Yang, Z., Zhang, L., Li, Y., and Pan, L., Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on g-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming, Int. J. Hydrogen Energy, 2017, vol. 42, pp. 9930–9937. https://doi.org/10.1016/j.ijhydene.2017.01.229
Águila, G., Jiménez, J., Guerrero, S., Gracia, F., Chornik, B., Quinteros, S., and Araya, P., A novel method for preparing high surface area copper zirconia catalysts. Influence of the preparation variables, Appl. Catal., A, 2009, vol. 360, pp. 98–105.
Sá, S., Hugo, S., Brandão, L., José, M.S., and Adélio, M., Catalysts for methanol steam reforming—a review, Appl. Catal., B, 2010, vol. 99, pp. 43–57.
Jones, S.D., Neal, L.M., and Hagelin-Weaver, H.E., Steam reforming of methanol using Cu–ZnO catalysts supported on nanoparticle alumina, Appl. Catal., B, 2008, vol. 84, pp. 631–642.
Conant, T., Karim, A.M., Lebarbier, V., Wang, Y., Girgsdies, F., Schlögl, R., and Datye, A., Stability of bimetallic Pd–Zn catalysts for the steam reforming of methanol, J. Catal., 2008, vol. 257, pp. 64–70.
Amjad, U., Vita, A., Galletti, C., Pino, L., and Specchia, S., Comparative study on steam and oxidative steam reforming of methane with noble metal catalysts, Ind. Eng. Chem. Res., 2013, vol. 52, pp. 15428–15436. https://doi.org/10.1021/ie400679h
Lytkina, A.A., Orekhova, N.V., Ermilova, M.M., Belenov, S.V., Guterman, V.E., Efimov, M.N., and Yaroslavtsev, A.B., Bimetallic carbon nanocatalysts for methanol steam reforming in conventional and membrane reactors, Catal. Today, 2016, vol. 268, pp. 60–67. https://doi.org/10.1016/j.cattod.2016.01.003
Wang, L.-C., Liu, Q., Chen, M., Liu, Y.-M., Cao, Y., He, H.-Y., and Fan, K.-N., Structural evolution and catalytic properties of nanostructured Cu/ZrO2 catalysts prepared by oxalate gel-coprecipitation technique, J. Phys. Chem., 2007, vol. 111, pp. 16549–16557. https://doi.org/10.1021/jp075930k
Lytkina, A.A., Orekhova, N.V., Ermilova, M.M., and Yaroslavtsev, A.B., The influence of the support composition and structure (MxZr1–xO2–d) of bimetallic catalysts on the activity in methanol steam reforming, Int. J. Hydrogen Energy, 2018, vol. 43, pp. 198–207. https://doi.org/10.1016/j.ijhydene.2017.10.182
Ockwig, N.W. and Nenoff, T.M., Membranes for hydrogen separation, Chem. Rev., 2007, vol. 107, no. 10, pp. 4078–4110.
Briceño, K., Montanè, D., Garcia-Valls, R., Iulianelli, A., and Basile, A., Fabrication variables affecting the structure and properties of supported carbon molecular sieve membranes for hydrogen separation, J. Membr. Sci., 2012, vol. 415, pp. 288–297. https://doi.org/10.1016/j.memsci.2012.05.015
Sazali, N., Mohamed, M.A., and Salleh, W.N.W., Membranes for hydrogen separation: a significant review, Int. J. Adv. Manuf. Technol., 2020, vol. 107, pp. 1859–1881. https://doi.org/10.1007/s00170-020-05141-z
Fernandez, E., Medrano, J.A., Melendez, J., Parco, M., Viviente, J.L., Van Sint Annaland, M., Gallucci, F., and Pacheco Tanaka, D.A., Preparation and characterization of metallic supported thin Pd–Ag membranes for hydrogen separation, Chem. Eng. J., 2016, vol. 305, pp. 182–190. https://doi.org/10.1016/j.cej.2015.09.119
Zhao, C., Sun, B., Jiang, J., and Xu, W., H2 purification process with double layer bcc-PdCu alloy membrane at ambient temperature, Int. J. Hydrogen Energy, 2020, vol. 45, pp. 17540–17547. https://doi.org/10.1016/j.ijhydene.2020.04.250
Ievlev, V.M., Prizhimov, A.S., and Dontsov, A.I., Structure of the α–β interface in a PdCu solid solution, Phys. Solid State, 2020, vol. 62, no. 1, pp. 59–64. https://doi.org/10.1134/S1063783420010138
Lee, S.M., Xu, N., Kim, S.S., Li, A., Grace, J.R., Lim, J.C., Boyd, T., Ryi, S.K., Susdorf, A., and Schaadt, A., Palladium/ruthenium composite membrane for hydrogen separation from the off-gas of solar cell production via chemical vapor deposition, J. Membr. Sci., 2017, vol. 541, pp. 1–8. https://doi.org/10.1016/j.memsci.2017.06.093
Petriev, I.S., Lutsenko, I.S., Voronin, K.A., Pushankina, P.D., and Baryshev, M.G., Hydrogen permeability of surface-modified Pd–Ag membranes at low temperatures, IOP Conf. Ser.: Mater. Sci. Eng., 2020, vol. 791, paper 012058.
Ievlev, V.M., Dontsov, A.I., Morozova, N.B., Roshan, N.R., Serbin, O.V., Prizhimov, A.S., and Solntsev, K.A., Techniques for surface cleaning of membrane foil from palladium-based solid solutions, Inorg. Mater., 2020, vol. 56, no. 10, pp. 1059–1064. https://doi.org/10.1134/S0020168520100052
Basov, N.L., Ermilova, M.M., Orekhova, N.V., and Yaroslavtsev, A.B., Membrane catalysis in the dehydrogenation and hydrogen production processes, Russ. Chem. Rev., 2013, vol. 82, no. 4, pp. 352–368. https://doi.org/10.1070/RC2013v082n04ABEH004324
Helmi, A., Fernandez, E., Melendez, J., Tanaka, D.A.P., Gallucci, F., and van Sint Annaland, M., Fluidized bed membrane reactors for ultra pure H2 production—a step forward towards commercialization, Molecules, 2016, vol. 21, paper 376. https://doi.org/10.3390/molecules21030376
Franchi, G., Capocelli, M., De Falco, M., Piemonte, V., and Barba, D., Hydrogen production via steam reforming: a critical analysis of MR and RMM, Membranes, 2020, vol. 10, paper 10. https://doi.org/10.3390/membranes10010010
Liguori, S., Iulianelli, A., Dalena, F., Piemonte, V., Huang, Y., and Basile, A., Methanol steam reforming in an Al2O3 supported thin pd-layer membrane reactor over Cu/ZnO/Al2O3 catalyst, Int. J. Hydrogen Energy, 2014, vol. 39, pp. 18702–18710. https://doi.org/10.1016/j.ijhydene.2013.11.113
Mironova, E.Yu., Lytkina, A.A., Ermilova, M.M., Efimov, M.N., Zemtsov, L.M., Orekhova, N.V., Karpacheva, G.P., Bondarenko, G.N., Yaroslavtsev, A.B., and Muraviev, D.N., Ethanol and methanol steam reforming on transition metal catalysts supported on detonation synthesis nanodiamonds for hydrogen production, Int. J. Hydrogen Energy, 2015, vol. 40, no. 8, pp. 3557–3565. https://doi.org/10.1016/j.ijhydene.2014.11.082
Piskin, F. and Öztürk, T., Combinatorial screening of Pd–Ag–Ni membranes for hydrogen separation, J. Membr. Sci., 2017, vol. 524, pp. 631–636. https://doi.org/10.1016/j.memsci.2016.11.066
Ievlev, V.M., Dontsov, A.I., Belonogov, E.K., Kannykin, S.V., and Solntsev, K.A., α ⇆ β phase transformations in rolled foil of the Pd–57 at % Cu solid solution, Inorg. Mater., 2017, vol. 53, no. 11, pp. 1163–1169. https://doi.org/10.1134/S0020168517110048
Stenina, I.A., Voropaeva, E.Yu., Veresov, A.G., Kapustin, G.I., and Yaroslavtsev, A.B., Effect of precipitation pH and heat temperature on the properties of hydrous zirconium dioxide, Russ. J. Inorg. Chem., 2008, vol. 53, no. 3, pp. 350–356. https://doi.org/10.1007/s11502-008-3002-0
Uluc, A.V., Moa, J.M.C., Terryn, H., and Bottger, A.J., Hydrogen sorption and desorption related properties of Pd-alloys determined by cyclic voltammetry, J. Electroanal. Chem., 2014, vol. 734, pp. 53–60. https://doi.org/10.1016/j.jelechem.2014.09.021
Wvarezza, R.C., Montemayor, M.C., and Fatas, E., Electrochemical study of hydrogen absorption in polycrystalline palladium, J. Electroanal. Chem., 1991, vol. 313, pp. 291–301.
Morozova, N.B., Vvedenskii, A.V., Maksimenko, A.A., and Dontsov, A.I., Thin layer multicycle cathodic–anodic chronoamperometry of atomic hydrogen injection–extraction into metals with regard to the stage of phase boundary exchange, Russ. J. Electrochemistry, 2018, vol. 54, no. 4, pp. 344–354. https://doi.org/10.1134/S1023193518040067
Stenina, I.A. and Yaroslavtsev, A.B., Interfaces in materials for hydrogen power engineering, Membr. Membr. Technol., Membr. Membr. Tekhnol., 2019, vol. 9, no. 3, pp. 137–144. https://doi.org/10.1134/S2517751619030065
Funding
This work was supported by the Russian Science Foundation (project no. 19-19-00232) and in part (synthesis and characterization of the catalyst) by the Russian Federation Ministry of Science and Higher Education (state research target for the Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Mironova, E.Y., Dontsov, A.I., Morozova, N.B. et al. Lamp Processing of the Surface of PdCu Membrane Foil: Hydrogen Permeability and Membrane Catalysis. Inorg Mater 57, 781–789 (2021). https://doi.org/10.1134/S0020168521080057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168521080057