Abstract
Owing to their high carbon and nitrogen contents, biogas residues may lead to higher carbon dioxide (CO2) and nitrous oxide (N2O) emissions from soils. Acidification of biogas slurry and application of nitrification inhibitors (NIs) could mitigate the emission of these gases. An incubation experiment was therefore carried out to investigate the effect of NIs, DMPP (3, 4-dimethylpyrazole phosphate), and PIADIN (active ingredients: 3.00–3.25% 1,2,4-triazole and 1.50–1.65% 3-methylpyrazole), on CO2 and N2O emissions from soils fertilized with biogas residues and acidified biogas residues. Biogas residues produced higher ammonium-nitrogen (NH4+-N) and nitrate-nitrogen (NO3−-N) concentrations in soils which resulted in higher emissions of CO2-C and N2O-N than that from acidified biogas residues. Both DMPP and PIADIN significantly decreased the emissions of CO2-C (8.1–55.8%) and N2O-N (87–98%) and maintained lower NH4+-N and NO3−-N concentrations when compared to control (without nitrification inhibitors). However, the DMPP had a higher reduction capability for CO2-C emissions than PIADIN in acidified biogas residue applied soil. In conclusion, the acidification of biogas residues and application of NIs are effect in reducing gaseous emission from biogas residue fertilized soils and thus could improve the fertilizer effectiveness of the residues.
Similar content being viewed by others
1 Introduction
Carbon dioxide (CO2) and nitrous oxide (N2O) are the primary greenhouse gases (GHGs) present in the Earth’s atmosphere (IPCC, 2010). CO2 could hang around for a long time, between 300 and 1000 years, once it is added to the atmosphere (Alan, 2019). The lifetime of atmospheric CO2 concentration, with an increase of about 120 ppm over the past 250 years, has risen to a current global average of approximately 409 ppm, and future rapid increase is expected, with values likely to reach 550 ppm by mid-century and 1000 ppm by the end of this century (IPCC, 2014). Similarly, N2O is a long-lived GHG, has a lifetime of 116 ± 9 years (Prather et al., 2015), and is a major stratospheric ozone-depleting substance (Thompson et al., 2019). Its concentration in the atmosphere has also risen steadily since the mid-twentieth century (IPCC, 2013), from approximately 290 ppb in 1940 to 330 ppb in 2017 (Park et al., 2012).
Agricultural practices, particularly the use of nitrogenous fertilizers, substantially contribute to enhancing N2O emission and thus increasing the concentration of reactive nitrogen (N, N2O) in the atmospheric environment (Bouwman et al., 2013). In soils, N2O is produced as a by-product of nitrification and denitrification processes which are carried out by different types of microbes (Bremner, 1997). Nitrification is the biological oxidation of NH4+ to nitrite (NO2−) and further to nitrate (NO3−). Denitrification is a microbially facilitated process in which NO3− is reduced, through a series of intermediate gaseous N oxide products, to molecular nitrogen (N2) (Köster et al., 2013). To meet the ambitious climate change adaptations, CO2 and N2O emissions should be minimized (IPPC, 2018).
Various techniques are used to reduce the CO2 and N2O emissions while meeting the growing demand for food and other agricultural products (Thompson et al., 2019). One of the approaches is to produce biogas through fermentation of renewable sources, including organic manures, plant materials, food waste, etc. The use of biogas as an energy source could reduce dependency on fossil fuels and is expected to have no or even a positive effect on the atmospheric greenhouse gas balance (Herrmann, 2013). Biogas production plays an important role in the European bio-energy supply and has increased rapidly in Germany in recent years. According to the Agency of Renewable Energies, there are 837 biogas production factories in the northern part of Germany. It is evident that the recycling of the biogas residue (BR) from such a large number of biogas production factories installed in the state of Schleswig–Holstein became a problem. Mostly BR is applied to soil as organic fertilizers (Köster et al., 2014; Koszel & Lorencowicz, 2015) and is considered an essential component of cropping systems due to its high fertilization value (Möller & Stinner, 2009). The application of BR as a fertilizer improved soil fertility, plants quality, and their immunity to biotic and abiotic stress agents (Kouřimská et al., 2012).
In addition to improving soil quality, the BR application to soil, due to their high carbon and ammonium content, may increase the CO2 and N2O emissions and NO3− leaching from the soils (Hennig & Gawor, 2012). N2O emissions and NO3− leaching from BR may be minimized by their acidification before soil application and by using nitrification inhibitors (NIs). While application of NIs can further reduce NO3− leaching from BR applied soils by suppressing autotrophic nitrification and subsequent denitrification, which will ultimately also reduce N2O emission. In fertilizer (mineral, slurry, and organic manure) applied to soil, the use of NIs has shown reduced N2O emissions (VanderZaag et al., 2011) and thereby increased N use efficiency of the applied fertilizers (Subbarao et al., 2006).
We hypothesized that (a) application of ABR to the soil will decrease soil pH and show lower CO2 and N2O emissions than the application of BR and (b) NI application to ABR-amended soil will further lower the gaseous emission from the soil. Keeping this in view, an incubation pot experiment was conducted to investigate CO2 and N2O emissions as well as NH4+ and NO3− dynamics in the soils amended with BR and ABR and applied with DMPP and PIADIN nitrification inhibitors.
2 Material and Methods
2.1 Collection and Preparation of Soil and Biogas Residues
The biogas residues (BR) were collected from a large commercial biogas company in Germany in May 2019. Its digesters were used to feed with the following materials: 18-t corn silage, 55-t dry chicken feces, 4-t whole crop silage (rye), and 7 m3 swine manure. For the incubation experiment, BR was sampled directly from the biogas residue storage pool. Acidified biogas residues (ABR) were prepared by lowering the pH of BR from 7.9 to 5.5 with the addition of H2SO4.
The soil was collected from an upper 20-cm layer of a natural grassland (which is adjacent to an agricultural farm and its soil has the same characters as that of agricultural soil) in Grevenkrug, Schleswig–Holstein (54° 11ʹ 09.1″ N and 10° 00ʹ 36.6″ E). The soil was sandy in texture, with 5% silt and 95% sand. The water-holding capacity (WHC) of the soil was 24.34% and bulk density was 1.4 g cm−3. The soil was air-dried and sieved through a 2-mm sieve to remove visible plant residues, roots, and stones. The total soil C and N contents in the soil were determined using a CN analyzer (Flash EA™ 1112, Thermo Fischer Scientific, Waltham, Massachusetts, USA). The fresh BR was got analyzed from Raiffeisen Laborservice (Ormont, Germany) for the salient characteristics. The salient characteristics of the soil and fresh BR are given in Table 1.
2.2 Treatments and Experimental Design
Three treatments of biogas residues (unamended, BR and ABR) were tested against three treatments of nitrification inhibitors (control, DMPP (3, 4-dimethylpyrazole phosphate), and PIADIN (active ingredients: 3.00–3.25% 1,2,4-triazole and 1.50–1.65% 3-methylpyrazole)), yielding 9 treatment combinations in total. The soil was filled in cylindrical pots (15-cm diameter and 33-cm length) up to 20-cm height while adjusting the bulk density to 1.4 g cm−3. Both BR and ABR were applied to the respective pots at the rate of 27.8 g kg−1 soil (equivalent to 0.1 g NH4+-N kg−1 soil), whereas unamended treatment did not receive any amendment. The DMPP and PIADIN were applied at the rate of 5 mg kg−1 soil (5% of the applied NH4+-N), whereas no NI was added to the control pots. The pots were incubated for 57 days in a climatic chamber at a constant temperature (15 °C), soil moisture (80% WHC), and air humidity (50%). Deionized water was added daily to maintain the desired soil moisture level. The experiment followed a two-factorial completely randomized design with four replicates.
2.3 Collection and Measurement of N2O and CO2
The N2O and CO2 samples were collected daily during 1st week, once after 2 days during the 2nd week, and once after 3 days during rest of the incubation period. Before collecting gas samples, the pots were tightly closed with air-tight lids having one rubber membrane, which served as a contact between the sample collecting syringe and the incubated environment. The samples were collected every 0, 20, 40, and 60 min. A 10-ml syringe with a hypodermic needle was placed in the pre-evacuated 2-ml headspace of Chromacol glass vials to collect the gas samples. The glass vials had a chloro-butyl rubber septum. Each gas sampling was carried out between 09:00 and 11:00 am. Except for the times when samples were being taken, the pots were left open. The concentrations of CO2 and N2O in the gas samples were measured by gas chromatography (Agilent 7890A GC, Agilent, CA, United States). An electron capture detector (ECD), adjusted to a temperature of 300 °C with N2 as a carrier gas, was used to measure N2O concentration. A thermal conductivity detector (TCD), adjusted to a temperature of 250 °C with He as a carrier gas, was used to measure CO2 concentration (Guo et al., 2021a). For each gas measurement, the gas chromatograph was calibrated with respective certified gas standards. The rate of CO2 and N2O emissions from each pot (ppm/min) during lid closure was calculated using headspace volume and a linear relation between the CO2 and N2O concentration and time (Venterea et al., 2020). The emission rates of CO2-C (μg h−1 kg−1) and of N2O-N (ng h−1 kg−1) were calculated with the following equations:
where ECO2 and EN2O are emission rates of CO2-C (μg h−1 kg−1) and N2O-N (ng h−1 kg−1), respectively; R is the rate of CO2 and N2O emissions from each pot (ppm/min); Vgas is the gas volume in pot (L); Wsoil is the weight of dry soil in pot (kg); AR is the relative atomic mass of C and N, i.e., 12 and 14, respectively; and Vm is the molar volume of gas which is 23.7 L/mol at 15 °C.
Total N2O and CO2 emissions during the incubation period were calculated by adding the total daily N2O and CO2 emissions. For this purpose, the emission rates of CO2-C (μg h−1 kg−1) and N2O-N (ng h−1 kg−1) were multiplied by the hours, i.e., 24 h for the 1st week, 48 h for the 2nd week, and 72 h for the last 6 weeks. The submission of all these gave the total emissions of CO2 (mg kg−1) and N2O (μg kg−1).
2.4 Soil Sampling and Measurement of NH4 +, NO3 −, and pH
For the measurement of NH4+-N, NO3—N, and pH, soil samples (each 50 g approx.) were collected up to 20-cm depth using a specialized soil agar on days 1, 15, 29, 43, and 57 of incubation. Each soil sample was divided into two subsamples; the first was oven-dried at 105 °C for 8 h to calculate the water content, while the other was used for the determination of NH4+-N, NO3−-N and pH. For soil mineral N analysis, 10-g fresh soil was extracted with 40 mL of 0.0125 M CaCl2 solution (1:4) for 1 h on a reciprocating shaker. The suspensions so obtained were centrifuged for 10 min, filtered through Whatman filter paper No. 42, and stored at 4 °C. The concentrations of NH4+ and NO3− were measured by a continuous flow autoanalyzer (San++ Automated Wet Chemistry Analyzer—Continuous Flow Analyzer (CFA), Skalar, The Netherlands). For pH measurement, 10-g air-dried and sieved soil was mixed with 25 mL of 0.0125 M CaCl2 solution (1:2.5) and shaken for 1 h. After centrifugation of the suspensions, the pH of the upper clear liquid was measured using a pH meter.
2.5 Statistical Analysis
Data were verified for normal distribution, treatment means for total N2O-N, and CO2-C emissions over the incubation period were compared using a two-way analysis of variance. pH over the different treatment at the same day was compared using a two-way analysis of variance. The significance of differences between individual treatment means was determined using Tukey’s honestly significant difference (HSD) test at P ≤ 0.05. Tukey’s HSD test was performed by R statistical software (Oakland, CA, USA). R was used to create the artwork.
3 Results
3.1 Rate of CO2-C and N2O-N Emissions
In unamended soil, the rates of CO2-C and N2O-N emissions were substantially lower than the biogas residues amended soils and there was no consistent pattern of increase or decrease with the passage of time during the incubation period (Figs. 1 and 2). However, rates of both CO2-C and N2O-N emissions were substantially higher in BR- and ABR-amended soils than unamended soil, with BR application showing more increase than ABR application. In BR- and ABR-amended soils, rates of emissions reached maximum by the end of the first or mid of the 2nd week, and thereafter decreased gradually, reaching the minimum value at the end of the incubation period. Application of DMPP and PIADIN to BR- and ABR-amended soils decreased the emissions rates of both gases.
3.2 Total CO2-C and N2O-N Emissions
Irrespective of whether NIs were applied or not, total CO2-C emission was very low from unamended soil than that from BR- and ABR-amended soils. The total CO2-C emission was the highest from BR-amended soil (169 mg kg−1 soil), followed by ABR-amended soil (81 mg kg−1 soil) and the lowest from unamended soil (37 mg kg−1 soil) (Table 2). Compared to unamended soil, the application of BR and ABR increased total CO2-C emission by 3.60- and 1.21-fold, respectively. Total N2O-N emission was also significantly higher from BR- and ABR-amended soils than that from unamended soil (Table 3). The mean total N2O-N emission was the highest from BR-amended soil (3678 μg kg−1), followed by ABR-amended soil (1464 μg kg−1), and the lowest from unamended soil (5 μg kg−1). Compared to unamended soil, the application of BR and ABR increased the total N2O-N emission by 73- and 29-folds, respectively.
ABR-amended soil showed half CO2-C emission of what did the BR-amended soil (Table 2). The NIs (DMPP and PIADIN) significantly lowered total CO2-C emission compared to control, but their relative effectiveness depended upon the residue types (Table 2). In BR-amended soil, DMPP and PIADIN were equally effective in reducing CO2-C emission, with reduction amounted to be 55% of that from control. However, in ABR-amended soil, DMPP was more effective in reducing CO2-C emission, showing a 38% decrease over control, than PIADIN which showed only an 8% decrease. Overall, the CO2-C emission-reducing capacity of both the NIs was higher in BR-amended soil than that in ABR-amended soil.
ABR-amended soil showed 60% lower total N2O-N emission than BR-amended soil (Table 3). The application of NIs further much lowered the total N2O-N emission compared to control, with DMPP having higher reduction efficiency than PIADIN under both BR- and ABR-amended soils. The mean reduction in total N2O-N emission in BR- and ABR-amended soil by DMPP and PIDIN was 96 and 90%, respectively.
3.3 Soil NH4 +-N and NO3 −-N Concentrations
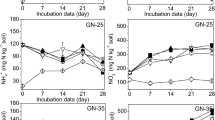
The application of both BR and ABR substantially increased NH4+-N and NO3−-N concentrations compared to control (Fig. 3). With the passage of time, ABR application showed a slow gradual decline in NH4+ concentration and a rise in NO3− concentration as compared to BR-amended soil. Similarly, the residue-amended soils applied with NIs showed a gradual decline in NH4+-N concentration and rise in NO3−-N concentration, whereas these changes were quite sharp in control soils. Moreover, DMPP more strongly retarded the fall in NH4+-N concentration and rise in NO3−-N concentration than PIADIN in both BR- and ABR-amended soils. The peak NO3−-N concentrations were lower in NI-treated soils as compared to control and DMPP produced the lowest NO3−-N concentration.
Time-dependent change in NH4+-N and NO3−-N concentrations in unamended, biogas residue (ABR)– and acidified biogas residue (ABR)–amended soils applied with control, DMPP, and PIADIN nitrification inhibitors under controlled environment. The vertical bars indicate the standard errors of the means (n = 4)
3.4 Soil pH
The application of BR did not affect the soil pH throughout the course of the experiment (Fig. 4). However, ABR application lowered the soil pH as compared to unamended soil and the mean decrease during the incubation period was 0.34 units. The application of DMPP and PIADIN had no influence on the soil pH.
Changes in pH of unamended, biogas residues (BR)- and acidified biogas residues (ABR)- amended soils applied with control, DMPP, and PIADIN nitrification inhibitors. The vertical bars indicate the standard errors of the means (n = 4). The values indicated with the same lowercase letter(s) on the same days are not significantly different at P = 0.05
4 Discussion
4.1 Effect of Biogas Residues
The application of biogas residues to agricultural soils as an organic fertilizer has become a common practice and is further growing with the increase in the number of biogas plants in some European countries (Köster et al., 2015; Wolf et al., 2014). The application of BR no doubt improves soil organic matter and N availability to crops, but on other sites, it can also provoke CO2 and N2O emissions from soils (Köster et al., 2011, 2015; Senbayram et al., 2014). Thus, high application rates of BR to cultivated lands could be linked to environmental problems such as high levels of CO2 and N2O in the atmosphere and NO3− leaching to groundwaters (Qu et al., 2014). This study revealed that BR application to soil significantly increased CO2 and N2O emissions (Figs. 1 and 2; Tables 2 and 3). A sharp decline in NH4+-N concentration and a rise in NO3−-N concentration was recorded in BR-amended soil (Fig. 3). Concurrent presence of NH4+, NO3−, and high level of labile C in the BR could have hastened the nitrification and denitrification processes in the soil (Jaeger et al., 2013) and thus increased CO2 emissions (Köster et al., 2015) and N2O emissions (Senbayram et al., 2009) compared with unamended soil. The availability of a high level of NO3− as a result of nitrification together with labile C under anaerobic soil conditions serves as a driving force for N2O emissions (Senbayram et al., 2009). Groffman and Crawford (2003) reported that CO2 efflux and denitrification activity can be positively correlated, and hence, increased denitrification activity also increases CO2 efflux. It is generally accepted that the application of organic fertilizers stimulates soil microbial biomass, basal respiration (Ros et al., 2003), and enzyme activities (Chu et al., 2007). Soil microbial processes also produce gases such as N2O (IPCC, 2013), and residual organic carbon substrates favor soil microbial denitrification (Robertson & Groffman, 2007). In conclusion, BR application to soil significantly increases CO2-C and N2O-N emissions from soils.
4.2 Acidification of BR and the Gaseous Emission
Soil N dynamics in BR-amended soils could depend on the BR composition and stability (Alburquerque et al., 2012). Hence, through changing the soil characteristics, BR may affect the nitrification process and hence the amounts of NO3− lost by denitrification or leaching (Peter et al., 2004). Among the soil characteristics, pH is a master variable that affect microbial transformations in soils. Nitrification is strongly influenced by pH (Mackens et al., 2021), with maximum rates occurring at pH 7.5 (Eric et al., 2002). Oxidation of NH4+ is completely inhibited at pH 5 and increases with a higher pH (Wang et al., 2018). Our result showed that application of ABR lowered soil pH than unamended soil, but this pH effect was not observed for BR-amended soil (Fig. 4). Accordingly, ABR application resulted in half CO2-C and 60% less N2O-N emissions from the soil compared to BR application (Tables 2 and 3). This is well explained by a slow gradual decline in NH4+ concentration and a rise in NO3− in ABR-amended soil as compared to BR-amended soil (Fig. 3). Thus, it could be inferred that ABR application retarded the nitrification process through a pH-driven shift in the microbial community structure and/or microbial activities (Ottosen et al., 2009). Fangueiro et al. (2013) also reported that lowering the pH led to decreased CO2 emission from soil, and the effect is due to the low microbial activities in the acidified soil (Fangueiro et al., 2015). In addition, since a small amount of H2SO4 could lower the pH of BR from 7.9 to 5.5, the cost of acidification is very low. In conclusion, ABR application to soil significantly decreases CO2-C and N2O-N emissions from soils compared to BR.
4.3 Nitrification Inhibitors and the Gaseous Emissions
Application of NIs is recognized as one of the mitigation strategies that have been proven to be highly effective in reducing N fertilizer losses, increasing N use efficiency, and crop yields under different cropping systems (Moir et al., 2012; Zhang et al., 2015). Therefore, we tested the compatibility of using the most common NIs DMPP and PIADIN with BR and ABR for reducing CO2 and N2O emission from soils. Our result showed that NIs reduced CO2 and N2O emissions in BR- and ABR-amended soil (Figs. 1 and 2; Tables 2 and 3). For reducing CO2 emission, the efficiency of NIs was relatively less in ABR-amended soil than BR-amended soil and DMPP was more effective than PIADIN only in ABR-amended soil (Table 2). However, NIs were equally effective in reducing N2O emission in BR- and ABR-amended soil, and DMPP was relatively more effective than PIADIN under both conditions (Table 3). The application of PIADIN has been reported to reduce N2O emissions by 37–62% during the weeks following biogas residue application to soil (Wolf et al., 2014). In fact, DMPP and PIADIN maintained NH4+-N concentration at a higher level which showed that NIs retarded the nitrification process and kept NO3−-N concentration at a low level (Fig. 3). Most NIs retard microbial oxidation of NH4+ by depressing the activities of nitrifiers in soil (Wolf et al., 2014). Thus, NIs could have inhibited nitrification through suppressing the activity of ammonia-oxidizing bacteria or relevant enzymes, effectively delaying the oxidization process that transforms NH4+ into NO3−. The decrease of CO2-C by applied NIs (Guo et al., 2021b) mostly was caused by the inhibition of microbial activities (Wolf et al., 2014). We found long-lasting higher NH4+-N concentration and lower CO2-C and N2O-N emissions from DMPP-treated soil as compared to PIADIN-treated soil (Fig. 3; Tables 2 and 3). This implies that DMPP was more effective than PIADIN in retarding the nitrification process and mitigating the gaseous emission from both BR- and ABR-amended soils under given experimental conditions. Chen et al. (2010) and Fangueiro et al. (2009) also reported that DMPP maintained the highest soil NH4+ content and a low soil NO3− content for a longer time than the other NIs and resulted in the highest reduction in N2O emissions.
5 Conclusion
Application of both BR and ABR substantially increased soil NH4+ content, but ABR-amended soil showed much lower CO2-C and N2O-N emissions than BR-amended soil. DMPP and PIADIN were equally effective in reducing CO2-C emission from BR-amended soil but DMPP was more effective than PIADIN in ABR-amended soil. DMPP almost completely diminished the N2O emission from both BR- and ABR-amended soils while the efficacy of PIADIN was relatively lower than DMPP in both cases. Acidification of BR did not further improve the efficacy of NIs, rather it had a slightly negative effect on the performance of PIADIN. Thus, it is concluded that although acidification of BR had an ameliorating effect on CO2 and N2O emissions from soils, it is not required when NIs have already been applied to the soils. However, acidification of BR could be beneficial in lowering N2O emission from the soils.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Change history
12 November 2021
A funding note has been added.
References
Alan, B. (2019). The atmosphere: Getting a handle on carbon dioxide. NASA’s jet propulsion laboratory. Humanity’s impacts on earth’s changing atmosphere: A five-part series. Global Climate Changes, 10(9). https://climate.nasa.gov/news/2915/the-atmosphere-getting-a-handle-on-carbon-dioxide/
Alburquerque, J. A., de la Fuente, C., & Bernal, M. P. (2012). Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agriculture, Ecosystems and Environment, 160, 15–22. https://doi.org/10.1016/j.agee.2011.03.007
Bouwman, A. F., Beusen, A. H. W., Griffioen, J., Van Groenigen, J. W., Hefting, M. M., Oenema, O., Van Puijenbroek, P. J. T. M., Seitzinger, S., Slomp, C. P., & Stehfest, E. (2013). Global trends and uncertainties in terrestrial denitrification and N2O emissions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 368, 20130112. https://doi.org/10.1098/rstb.2013.0112
Bremner, J. M. (1997). Sources of nitrous oxide in soils. Nutrient Cycling in Agroecosystems, 49, 7–16. https://doi.org/10.1023/A:1009798022569
Chen, D., Suter, H. C., Islam, A., & Robert, E. (2010). Influence of nitrification inhibitors on nitrification and nitrous oxide N2O emission from a clay loam soil fertilized with urea. Soil Biology and Biochemistry, 42(4), 660–664. https://doi.org/10.1016/j.soilbio.2009.12.014
Chu, H. Y., Lin, X. G., Fujii, T., Morimoto, S., Yagi, K., Hu, J., & Zhang, J. (2007). Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biology and Biochemistry, 39, 2971–2976. https://doi.org/10.1016/j.soilbio.2007.05.031
Eric, A. S., Nicole, L. M., & Gary, A. L. (2002). Factors regulating nitrification in aquatic sediments: Effects of organic carbon, nitrogen availability, and pH. Canadian Journal of Fisheries and Aquatic Sciences, 59(3), 554–563. https://doi.org/10.1139/f02-032
Fangueiro, D., Fernandes, A., Coutinho, J., Moreira, N., & Trindade, H. (2009). Influence of two nitrification inhibitors (DCD and DMPP) on annual ryegrass yield and soil mineral N dynamics after incorporation with cattle slurry. Communications in Soil Science and Plant Analysis, 40, 3387–3398. https://doi.org/10.1080/00103620903325976
Fangueiro, D., Hjorth, M., & Gioelli, F. (2015). Acidification of animal slurry—A review. Journal of Environmental Management, 149, 46–56. https://doi.org/10.1016/j.jenvman.2014.10.001
Fangueiro, D., Surgy, S., Coutinho, J., & Vasconcelos, E. (2013). Impact of cattle slurry acidification on carbon and nitrogen dynamics during storage and after soil incorporation. Journal of Plant Nutrition and Soil Science, 176, 540–550. https://doi.org/10.1002/jpln.201200117
Groffman, P. M., & Crawford, M. K. (2003). Denitrification potential in urban riparian zones. Journal of Environmental Quality, 32, 1144–1149. https://doi.org/10.2134/jeq2003.1144
Guo, Y., Becker-Fazekas, S., & Mühling, K. H. (2021a). Impact of different chloride salts and their concentrations on nitrification and trace gas emissions from a sandy soil under a controlled environment. Soil Use and Management. https://doi.org/10.1111/sum.12713
Guo, Y., Naeem, A., & Mühling, K. H. (2021b). Comparative effectiveness of four nitrification inhibitors for mitigating carbon dioxide and nitrous oxide emissions from three different textured soils. Nitrogen, 2, 155–166. https://doi.org/10.3390/nitrogen2020011
Hennig, C., & Gawor, M. (2012). Bioenergy production and use: Comparative analysis of the economic and environmental effects. Energy Conversion and Management, 63, 130–137. https://doi.org/10.1016/j.enconman.2012.03.031
Herrmann, A. (2013). Biogas production from maize: Current state, challenges and prospects. 2. Agronomic and environmental aspects. Bioenergy Research, 6, 372–387. https://doi.org/10.1007/s12155-012-9227-x
IPCC. (2010). Contribution of working group I to the third assessment report of the Intergovernmental Panel on Climate Change. In J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, X. Dai, K. Maskell, C. A. Johnson (Eds.). Climate change 2001: The scientific basis. Cambridge University Press.
IPCC (2013). Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In T.F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (Eds.). Climate Change 2013: The Physical Science Basis.1535. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA,
IPCC. (2014). Summary for policymakers. In C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, M. Chatterjee, K. L. Ebi, Y. O. Estrada, R. C. Genova, B. Girma, E. S. Kissel, A. N. Levy, S. MacCracken, P. R. Mastrandrea, & L. L. White (Eds.), Climate change 2014: Impacts, adaptation, and vulnerability, Part A: Global and sectoral aspects. Contribution of Working Group II to the 5th Assessment Report of the Intergovernmental Panel on Climate Change (pp. 1–32). Cambridge University Press.
IPPC, et al. (2018). Summary for policymakers. In V. Masson-Delmotte (Ed.), Global warming of 15 °C. World Meteorological Organization.
Jaeger, N., Duffner, A., Ludwig, B., & Flessa, H. (2013). Effect of fertilization history on short-term emission of CO2 and N2O after the application of different N fertilizers-a laboratory study. Archives of Agronomy and Soil Science, 59(2), 161–171. https://doi.org/10.1080/03650340.2011.621420
Köster, J. R., Cárdenas, L., Senbayram, M., Bol, R., Well, R., Butler, M., Mühling, K. H., & Dittert, K. (2011). Rapid shift from denitrification to nitrification in soil after biogas residue application as indicated by nitrous oxide isotopomers. Soil Biology and Biochemistry, 43, 1671–1677. https://doi.org/10.1016/j.soilbio.2011.04.004
Köster, J. R., Cárdenas, L. M., Bol, R., Lewicka-Szczebak, D., Senbayram, M., Well, R., Giesemann, A., & Dittert, K. (2015). Anaerobic digestates lower N2O emissions compared to cattle slurry by affecting rate and product stoichiometry of denitrification—An N2O isotopomer case study. Soil Biology and Biochemistry, 84, 65–74. https://doi.org/10.1016/j.soilbio.2015.01.021
Köster, J. R., Dittert, K., Mühling, K. H., Kage, H., & Pacholski, A. (2014). Cold season ammonia emissions from land spreading with anaerobic digestates from biogas production. Atmospheric Environment, 4, 35–38. https://doi.org/10.1016/j.atmosenv.2013.11.037
Köster, J. R., Well, R., Dittert, K., Giesemann, A., Lewicka-Szczebak, D., Mühling, K. H., Herrmann, A., Lammel, J., & Senbayram, M. (2013). Soil denitrification potential and its influence on the N2O reduction and N2O isotopomer ratios. Rapid Communications in Mass Spectrometry, 27, 2363–2373. https://doi.org/10.1002/rcm.6699
Koszel, M., & Lorencowicz, E. (2015). Agricultural use of biogas digestate as a replacement fertilizer. Agriculture and Agricultural Science Procedia, 7, 119–124. https://doi.org/10.1016/j.aaspro.2015.12.004
Kouřimská, L., Poustková, I., & Babička, L. (2012). The use of digestate as a replacement of mineral fertilizers for vegetables growing. Scientia Agriculturae Bohemica, 43(4), 121–126. https://doi.org/10.7160/sab.2012.430401
Mackens, J. T., Görlach, B. M., & Mühling, K. H. (2021). Acidified biogas residues improve nutrient uptake and growth of young maize. Agronomy, 11, 344. https://doi.org/10.3390/agronomy11020344
Moir, J. L., Malcolm, B. J., Cameron, K. C., & Di, H. J. (2012). The effect of dicyandiamide on pasture nitrate concentration, yield and N offtake under high N loading in winter and spring. Grass and Forage Science, 67, 391–402. https://doi.org/10.1111/j.1365-2494.2012.00857
Möller, K., & Stinner, W. (2009). Effects of different manuring systems with and without biogas digestion on soil mineral nitrogen content and on gaseous nitrogen losses (ammonia, nitrous oxides). European Journal of Agronomy, 30, 1–16. https://doi.org/10.1016/j.eja.2008.06.003
Ottosen, L. D. M., Poulsen, H. V., Nielsen, D. A., Finster, K., Nielsen, L. P., & Revsbech, N. P. (2009). Observations on microbial activity in acidified pig slurry. Biosystems Engineering, 102(3), 291–297. https://doi.org/10.1016/j.biosystemseng.2008.12.003
Park, S., Croteau, P., Boering, K. A., Etheridge, D. M., Ferretti, D., Fraser, P. J., Kim, K. R., Krummel, P. B., Langenfelds, R. L., van Ommen, T. D., Steele, L. P., & Trudinger, C. M. (2012). Trends and seasonal cycles in the isotopic composition of nitrous oxide since 1940. Nature Geoscience, 5, 261–265. https://doi.org/10.1038/ngeo1421
Peter, M. K., Alfred, M. B., Jason, W. E., & Ramon, I. (2004). Soil pH effects on nitrification of fall-applied anhydrous ammonia. Soil Science Society of America Journal, 68, 545–551. https://doi.org/10.2136/sssaj2004.5450
Prather, M. J., Hsu, J., DeLuca, N. M., Jackman, C. H., Oman, L. D., Douglass, A. R., Fleming, E. L., Strahan, S. E., Steenrod, S. D., Søvde, O. A., Isaksen, I. S. A., Froidevaux, L., & Funke, B. (2015). Measuring and modeling the lifetime of nitrous oxide including its variability. The Journal of Geophysical Research: Atmospheres, 120, 5693–5705. https://doi.org/10.1002/2015JD023267
Qu, Z., Wang, J., Almøy, T., & Bakken, L. R. (2014). Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils. Global Change Biology, 20(5), 1685–1698. https://doi.org/10.1111/gcb.12461
Robertson, G. P., & Groffman, P. M. (2007). Nitrogen transformations. In E. A. Paul (Ed.), Soil microbiology, ecology, and biochemistry (pp. 341–364). Springer. https://doi.org/10.1016/B978-0-08-047514-1.50017-2
Ros, M., Hernandez, M. T., & Garcı́a, C. (2003). Soil microbial activity after restoration of a semiarid soil by organic amendments. Soil Biology and Biochemistry, 35(3), 463–469. https://doi.org/10.1016/S0038-0717(02)00298-5
Senbayram, M., Chen, R., Mühling, K. H., & Dittert, K. (2009). Contribution of nitrification and denitrification to nitrous oxide emissions from soils after application of biogas waste and other fertilizers. Rapid Communications in Mass Spectrometry, 23, 2489–2498. https://doi.org/10.1002/rcm.4067
Senbayram, M., Chen, R., Wienforth, B., Herrmann, A., Kage, K., Mühling, K. H., & Dittert, K. (2014). Emission of N2O from biogas crop production systems in Northern Germany. BioEnergy Research, 7, 1223–1236. https://doi.org/10.1007/s12155-014-9456-2
Subbarao, G. V., Ito, O., Sahrawat, K. L., Berry, W. L., Nakahara, K., Ishikawa, T., Watanabe, T., Suenaga, K., Rondon, M., & Rao, I. M. (2006). Scope and strategies for regulation of nitrification in agricultural systems-challenges and opportunities. Critical Reviews in Plant Sciences, 25(4), 303–335. https://doi.org/10.1080/07352680600794232
Thompson, R. L., Lassaletta, L., Patra, P. K., Wilson, C., Wells, K. C., Gressent, A., Koffi, E. N., Chipperfield, M. P., Winiwarter, W., Davidson, E. A., Tian, H., & Canadell, J. G. (2019). Acceleration of global N2O emissions seen from two decades of atmospheric inversion. Nature Climate Change, 9, 993–998. https://doi.org/10.1038/s41558-019-0613-7
VanderZaag, A. C., Jayasundara, S., & Wagner-Riddle, C. (2011). Strategies to mitigate nitrous oxide emissions from land applied manure. Animal Feed Science and Technology, 166–67, 464–479. https://doi.org/10.1016/j.anifeedsci.2011.04.034
Venterea, R. T., Petersen, S. O., de Klein, C. A. M., Pedersen, A. R., Noble, A. D. L., Rees, R. M., Gamble, J. D., & Parkin, T. B. (2020). Global research alliance N2O chamber methodology guidelines: Flux calculations. Journal of Environmental Quality, 49, 1141–1155. https://doi.org/10.1002/jeq2.20118
Wang, J., Zhang, B. B., Tian, Y., Zhang, H., Cheng, Y., & Zhang, J. (2018). A soil management strategy for ameliorating soil acidification and reducing nitrification in tea plantations. European Journal of Soil Biology, 88, 36–40. https://doi.org/10.1016/j.ejsobi.2018.06.001
Wolf, U., Fuß, R., Höppner, F., & Flessa, H. (2014). Contribution of N2O and NH3 to total greenhouse gas emission from fertilization, results from a sandy soil fertilized with nitrate and biogas digestate with and without nitrification inhibitor. Nutrient Cycling in Agroecosystems, 100, 121–134. https://doi.org/10.1007/s10705-014-9631-z
Zhang, M., Fan, C. H., Li, Q. L., Li, B., Zhu, Y. Y., & Xiong, Z. Q. (2015). A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system. Agriculture, Ecosystems and Environment, 201, 43–50. https://doi.org/10.1016/j.agee.2014.12.003
Acknowledgements
Ahmad Khan is thankful to the Alexander von Humboldt Foundation for a re-invitation grant for a research visit at Kiel University.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the funding of the Ph.D. project by the Society of Energy and Climate (EKSH) of Schleswig–Holstein (14/12–24) and the Alexander von Humboldt Foundation through the grant of George Forster Post-Doctorate Fellowship.
Author information
Authors and Affiliations
Contributions
YG: methodology, software, writing—original draft; AA: methodology, writing—review and editing; AK: methodology, writing—review and editing; AN: writing—review and editing; KHM: conceptualization, writing—review and editing, funding acquisition, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, Y., Anjum, A., Khan, A. et al. Comparative Effectiveness of Biogas Residue Acidification and Nitrification Inhibitors in Mitigating CO2 and N2O Emissions from Biogas Residue-Amended Soils. Water Air Soil Pollut 232, 345 (2021). https://doi.org/10.1007/s11270-021-05282-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05282-1