Abstract
Purpose of Review
Mitral valve repair is a common surgical procedure for both primary and secondary mitral regurgitation. With operations performed earlier in disease progression and increased patient longevity, the need for a repeat intervention is not infrequent. With the associated risks of reoperation and patient comorbidities, percutaneous techniques for acute or delayed failure after ring annuloplasty are emerging.
Recent Findings
Current commercially available devices, used in “off-label” ways, such as the MitraClip, may be effective in repairing recurrent mitral regurgitation after annuloplasty. Similarly, a valve-in-ring transcatheter mitral valve replacement can be considered in patients at high risk for surgical reoperation. These procedures are not without risk, for example, resultant mitral stenosis in the setting of edge-to-edge repair or left ventricular outflow tract (LVOT) obstruction with valve-in-ring transcatheter mitral valve replacement. Newer devices are emerging to permit more options for this subset of patients, which include transcatheter valves that are specifically designed for the mitral position.
Summary
Undoubtedly, surgical reoperation has increased risk as compared to primary operation. Though percutaneous options are evolving, use in this patient population is currently limited to “off-label” use and is also associated with procedural complexities and risk. It is prudent for cardiologists, surgeons, and anesthesiologists to weigh risks, benefits, and limitations when considering patients for surgical reoperation, percutaneous repair, or transcatheter replacement after failed mitral annuloplasty.
Similar content being viewed by others
Introduction
Mitral regurgitation (MR) affects more than 2 million people in the USA [1], with more than 44,000 patients who undergo surgical mitral valve surgery annually [2]. Mitral valve repair is the gold standard for treatment of MR, especially in degenerative valve disease. Compared to mitral valve replacement (MVR), mitral valve repair has a lower operative mortality rate and a 30-day mortality rate that is half of MVR [3].
Types of Mitral Valve Annuloplasty
According to 2014 AHA/ACC valvular heart disease guidelines, mitral valve repair is preferred over replacement if a successful and durable repair is achieved [3]. The type of leaflet repair is influenced by patient characteristics, e.g., the specific location and mechanism of the pathology within the mitral valvular apparatus, and surgical preferences, e.g., leaflet resection versus preservation [4]. For these repairs, annuloplasty rings are sutured into the annulus with the aim to restore physiologic form and preserve native annular dynamics [5]. Today, commercially available annuloplasty devices include flexible, semi-rigid, rigid, complete, partial, saddle, and planar-shaped rings. The specific prosthesis chosen is based on the pathophysiology of the valvular apparatus, risk of post-operative systolic anterior motion of the mitral valve, annular size and the extent of remodeling, and surgeon preference. Goals of surgical repair are to restore leaflet coaptation depth to more than 5 millimeters (mm), stabilize and remodel the annulus, restore normal leaflet motion, and eliminate regurgitation [6]. Given advancements in repair techniques and surgical expertise, patients have had excellent clinical outcomes with initial success rates for isolated mitral valve repair for primary MR greater than 75% [7], with certain institutions reporting initial success rates as high as 99% and rates of freedom from severe MR at 8 years of 97% [8].
Failure of Annuloplasty
Durability of repair in degenerative MR is typically long-lasting, with a freedom of reoperation rate of 80% and recurrent MR rate of 60% at 15 to 20 years [3]; however, recurrence of MR and annuloplasty failure still occurs. Likewise in functional MR, high-grade recurrent or residual MR after annuloplasty is relatively uncommon immediately postoperatively; however, the rate of regurgitation increases up to 28% in the first 6 months postoperatively [9]. Below, we will discuss acute and delayed causes and implications of mitral annuloplasty failure.
Regurgitation
Systolic Anterior Motion
Systolic anterior motion (SAM) of the mitral valve can range from minor chordal protrusion with little to no hemodynamic effects to severe left ventricular outflow obstruction with severe MR [10]. Intraoperative SAM after mitral valve repair is uncommon, with an incidence of less than 10% [11]. Only 2.3% of intraoperative SAM results in persistent SAM requiring immediate revision or repair after initial medical management with beta blockade, vasoconstriction, and volume expansion [11]. Brown et al. reported no late operations were required to correct SAM suggesting that prompt intraoperative diagnosis and medical management can relieve outflow obstruction to avoid both early and delayed interventions [11]. Medical management to improve hemodynamics includes increased afterload, decreased heart rate and contractility, and avoidance of inotropic medications. Alfieri et al. reported resolution of SAM after mitral valve repair in one-third of patients after volume expansion and discontinuation of inotropic agents, with about 80% resolving after the addition of beta blockade and afterload augmentation [10]. These low intervention rates could be attributed to the fact that SAM with left ventricular outflow obstruction is less common than isolated SAM causing negligible hemodynamic compromise, 20% versus 80% respectively [11]. SAM may occur late after mitral valve repair, but it is uncommon and can be corrected with surgical reoperation, percutaneously, or medical management. Kehl et al. described a single-center experience using disopyramide to successfully treat postoperative SAM [12]. In their cohort of seven patients, SAM was not observed intraoperatively, but rather a LVOT gradient was noted between 3 days postoperatively and even up to 20 months in two patients. In their series, all patients with new onset, severe SAM were initially treated with beta blockade. Five patients had persistent SAM despite maximal beta blockade and were treated with disopyramide, which ultimately led to significant improvement or resolution in LVOT obstruction and SAM.
Degeneration or Progression of Disease
Progression of native valve disease can occur after mitral valve repair and cause recurrent MR, especially if the primary repair was caused by degenerative valve disease. This is often due to newly prolapsed valve segments secondary to chordal pathology. There is a linear recurrence of 2–4% per year after degenerative mitral valve repair [13], with rates unsurprisingly higher in Barlow’s disease compared to mild fibroelastic deficiency. In patients with functional MR with ischemic or nonischemic cardiomyopathy who have undergone restrictive mitral valve annuloplasty, predictors for recurrent MR include distal leaflet tethering and posterior leaflet tethering [14]. After annuloplasty for functional MR in patients with ischemic cardiomyopathy, some will exhibit postoperative reverse remodeling of the left ventricle; however, patients without reverse remodeling of the left ventricle are more likely to have recurrent MR and increased mortality [15].
Stenosis
Compared to recurrent MR, mitral stenosis after valve repair is underreported in the literature as a marker for annuloplasty failure. In a retrospective analysis, factors that are associated with reoperation for mitral stenosis after mitral valve repair include smaller ring sizes and inflammatory or calcific changes within the valve with a median time to reoperation of 4.5 years after primary repair [16].
Therapeutic Options for Failed Annuloplasty
Surgical
Despite emerging percutaneous techniques, in patients who are surgical candidates, surgical reoperation remains the standard of care for degenerated bioprostheses and failed annuloplasty rings [17, 18]. Regardless of disease etiology, reoperation should always be considered as it has shown favorable results for overall survival and freedom from reoperation [19]. In a retrospective analysis comparing redo mitral valve repair versus replacement after prior mitral valve repair, patients in the redo mitral valve repair cohort had 0% operative mortality with 96% freedom from mortality at 1 year and 78% at 5 years, higher than the mitral valve replacement cohort [20].
Percutaneous
Transcatheter percutaneous approaches have emerged as options for patients with prohibitive surgical risk and remain symptomatic despite goal-directed medical management of heart failure [3]. Use in patients with functional MR has resulted in early and sustained health status improvement compared to medical therapy alone [21]. With percutaneous techniques constantly evolving, these interventions will likely emerge as the preferred and safest treatment options for many patients. In fact, recent case reports and limited retrospective analyses have shown promise for the future of percutaneous options after surgical bioprostheses degeneration or failure of surgical annuloplasty [22].
MitraClip
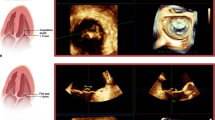
The MitraClip® (Abbott Laboratories, Abbott Park, IL, USA) debuted in 2008 and was later approved by the Food and Drug Administration (FDA) in the USA for patients at high surgical risk with degenerative MR. In the last few years, the MitraClip has emerged as a novel option for high-risk patients who would otherwise necessitate surgical re-repair or replacement for recurrent MR (Fig. 1A). Currently, there are no large-scale reports or clinical trials for the use of MitraClip after failed annuloplasty, but success of “off-label” use has been described in the literature.
A Three-dimensional transesophageal echocardiographic (TEE) assessment of a MitraClip (yellow arrow) in a partial mitral valve annuloplasty ring. B Three-dimensional TEE assessment of a complete D-shaped mitral valve annuloplasty ring (blue arrow) prior to valve-in-ring deployment. C Color flow Doppler three-dimensional TEE assessment of paravalvular leak (yellow arrow) after transcatheter mitral valve-in-ring, note the distorted ovoid shape of the transcatheter valve (blue arrow). D Three-dimensional TEE showing delivery wire of an Amplatzer vascular plug (yellow arrow) for paravalvular leak closure after transcatheter mitral valve-in-ring.
Grasso et al. conducted a prospective study of percutaneous mitral valve repair with MitraClip. This European study enrolled 154 consecutive patients with moderate-severe or severe MR who were determined to be at high surgical risk. In their study population, six patients had previously undergone surgical annuloplasty with a median time interval between surgery and percutaneous repair of 8 years; however, one percutaneous repair was 7 days after initial surgical repair. After MitraClip placement, all patients had less than or equal to moderate MR, and no patients had procedural death, stroke, and myocardial infarction or necessitated urgent surgical intervention. All patients had satisfactory gradients and mitral valve areas. The patient in which the percutaneous repair was performed in the acute phase after surgical repair died from multiorgan failure during their hospital stay. Of the remaining five patients, all but one had improvements in New York Heart Association (NYHA) functional class and maintenance or improvement in degree of regurgitation at a median 12-month follow-up [23]. This limited analysis of use in patients with annuloplasty failure demonstrates early success; however, larger series with long-term follow-up are needed.
As mentioned previously, SAM presenting late after mitral valve repair is uncommon. However, a case report of the first in-man MitraClip to treat SAM 1-year after mitral valve repair was demonstrated to be effective in alleviating LVOT obstruction by anchoring the anterior leaflet to the posterior leaflet, thereby preventing entrainment of the anterior leaflet into the left ventricular outflow tract during systole. In this report, in the immediate postprocedural period, there was no MR and at 6-month follow-up, exercise echocardiography showed no residual MR, reduced dynamic LVOT obstruction, and moderate stenosis of the valve [24]. This report suggests that the MitraClip system can be efficacious in cases of SAM after repair, but carries the possible risk of stenosis, which may be more pronounced in patients with previous leaflet resection or smaller annuloplasty rings.
Use of the MitraClip system has also been described in cases of ring dehiscence, which displayed marked improvement in degree of regurgitation and heart failure symptoms after placement of a single clip with durable results at 1-month follow-up [25].
Valve in Ring
Currently, valves used for transcatheter mitral valve replacement were designed to be used in the aortic position and anchor by expanding within a circular geometry [22]. This is contrary to the mitral annulus which is described as D-shape. Regardless of annuloplasty ring type, these prostheses are shaped to conform to the natural structural shape of the annulus (Fig. 1B). It is suggested that semi-rigid rings may be the ideal prosthetic to anchor a transcatheter valve as its flexibility permits conformation of the new valve but is rigid enough to provide adequate scaffolding [26], whereas rigid rings provide an adequate scaffolding, and they pose the greatest risk for paravalvular leak and percutaneous valve distortion (Fig. 1C) [22]. Similarly, partial rings, which are open anteriorly, have also been shown to be more susceptible to paravalvular regurgitation [27]. In a retrospective analysis of valve-in-valve and valve-in-ring replacements, paravalvular leak closure was more frequent in valve-in-ring patients, 6.9% versus 2.3%, respectively (Fig. 1D) [28]. Further, moderate or greater MR was more frequent in patients with flexible rings compared with semi-rigid rings, 44.4% versus 10.8%, implying specific challenges unique to the prior annuloplasty prostheses. Digital applications have been developed to assist with both assessment of suitability of a valve in ring procedure and device sizing and selection (valve-in-valve-mitral) [29].
In 2011, de Weger et al. reported the first in-human transcatheter mitral valve-in-ring with a transcatheter Sapien-Edwards aortic valve (Edwards Lifesciences Inc., Irvine, CA, USA) through a transapical approach [30]. This case report established the feasibility of using this transcatheter valve as an alternative to surgical valve replacement in patients at high risk for reoperation. The transapical approach remains the first choice for patients with peripheral vascular disease; however, the transfemoral transseptal delivery approach has the advantage of being percutaneous, avoids the negative impact on apical myocardial function, and may be performed under local anesthesia with or without sedation [31, 32].
The transcatheter mitral valve replacement registry is an international, multicenter, observational study that enrolled consecutive patients with degenerated mitral bioprostheses and failed annuloplasty rings that underwent transcatheter replacement [28]. Primary end points for this study were all-cause mortality at 30 days and 1 year. The valve-in-ring group had a 30-day all-cause mortality of 8.3%, which increased to 28.7% by 1 year. This may be in part due to selection bias, as these patients are more likely to be deemed poor surgical candidates given functional status or comorbid conditions and therefore present for transcatheter procedures as a “last resort” option. A 2018 systematic review and meta-analysis found that transcatheter mitral valve-in-valve or valve-in-ring procedures have a success rate of 93.5% and an in-hospital mortality of 5.8% when including both transseptal and transapical techniques [31]. As risk and benefits are considered for surgical reoperation, transcatheter replacement may be the most suitable and safest option for many aging patients with annuloplasty failure.
Similar to surgical mitral valve repair, LVOT obstruction is a potential complication after transcatheter replacement. Unique to transcatheter replacement of the mitral valve is the concept of the “neo-LVOT,” which is described as the elongation of the native LVOT by the downward displacement of the anterior leaflet of the mitral valve (Fig. 2B) [33]. Factors associated with an increased risk of LVOT obstruction include valve protrusion into the left ventricle, a long anterior mitral leaflet, device flaring, shallow aortomitral angle, and septal bulging. As described by Blanke et al. [33], a preprocedural three-dimensional multidetector computed tomography (MDCT) is the gold standard for pre-procedural imaging. Using multiplanar reconstruction of the MDCT enables annular and ring measurements to facilitate appropriate device sizing and can take into account the potential impact of pannus formation and any associated calcification. In addition, MDCT facilitates assessment of anatomical landmarks, the preexisting prosthesis, and projection of a virtual cylinder to simulate the transcatheter valve can estimate the “neo-LVOT” to predict those at risk for obstruction of the outflow tract (Fig. 2A) [33]. Observational data suggests a neo-LVOT area less than 190 mm2 predicts increased risk of LVOT obstruction [34]. Laceration of the anterior mitral valve leaflet to prevent outflow obstruction (LAMPOON) during transcatheter mitral valve replacement was studied in patients at prohibitive risk for surgical replacement with predicted LVOT obstruction or transcatheter heart valve dysfunction (Fig. 2C) [35]. This transcatheter procedure lacerates the A2 scallop of the anterior mitral leaflet immediately before transcatheter mitral valve deployment, thereby reducing the LVOT obstruction that would have been caused by an intact anterior leaflet (Fig. 2D) [36]. The LAMPOON IDE trial was a prospective, single-arm, multicenter study of the LAMPOON procedure with a transseptal Edwards Sapien 3 valve in annuloplasty ring or band, or native mitral annular calcification. Results of their study proved the efficacy and safety of the LAMPOON procedure in this particular patient population [36].
A Computed tomographic multiplanar reconstruction of left ventricular outflow tract (LVOT) (yellow arrow) with virtual transcatheter valve (blue arrow) inserted into annuloplasty ring, the short axis view is used to approximate the “neo-LVOT.” B Transesophageal echocardiographic (TEE) deep transgastric long axis assessment showing displacement of the anterior mitral leaflet (yellow arrow) into the LVOT causing obstruction and an associated peak gradient of 34 mmHg. Color flow Doppler demonstrates turbulent flow proximal to the aortic valve (AoV) (magenta arrow). C Three-dimensional TEE during a LAMPOON procedure showing a cautery wire at the base of A2 of the anterior mitral leaflet (blue arrow) and through the mitral valve orifice (yellow arrow) with the anticipated laceration of A2 (white dashed line). D Color flow Doppler TEE assessment of LVOT in the midesophageal aortic valve long axis view after LAMPOON procedure. There is minor flow acceleration due to blood flow through the cells of the valvular stent (blue arrow), but with no elevation in aortic valve peak gradient (11mmHg).
Investigational Devices (Altavalve, Intrepid Valve)
Recently, the AltaValve (4C Medical Technologies, Minneapolis, MN, USA) materialized as a new transcatheter mitral valve. It consists of a self-expanding nitinol frame, spherical in shape which is sized to fit into the left atrium. It contains a 27-mm trileaflet bovine pericardium valve. A fabric skirt wraps around the lower nitinol frame to minimize or prevent paravalvular leaks. This valve is designed to sit supra-annular to minimize LVOT obstruction and interaction with the subvalvular apparatus. The first in-person use of this valve was in 2019 and done via transapical approach under transesophageal echocardiography guidance [37]. Similar to other transcatheter valves, a transseptal delivery system option is under development. The Intrepid Valve (Medtronic, Minneapolis, MN, USA) is a transcatheter mitral valve replacement system that integrates a self-expanding 27-mm stented valve within a larger outer stent which is designed to engage with the mitral valve annulus and leaflets. This separates the bioprosthetic valve from interacting with the variable, noncircular, annular anatomy. Early feasibility in primary MR looks promising [38]. The APOLLO trial (https://clinicaltrials.gov/ct2/show/NCT03242642) has a single-arm cohort for patients with prohibitive risk and failed surgical prosthesis, and the results of these trials may add to the therapeutic options in this patient population.
Mitral Stenosis After Annuloplasty and Percutaneous Options
Mitral stenosis following mitral valve repair is not uncommon [39, 40], with as many as 50% of patients have mean gradients > 5mmHg and MVA < 1.5 cm2. In these patients, as many as 17% will require reoperation [39]. The mechanisms for stenosis are pannus ingrowth (57%) and leaflet calcification (34%) [16]. In these patients, percutaneous options are primarily limited to primarily valve in ring as described previously. Specific attention should be paid to the pre-operative CT scan to assess the potential impact of leaflet calcium or pannus on the transcatheter valve. Though uncommon, a successful rescue percutaneous balloon mitral valvuloplasty was reported in a case of acutely progressive cardiogenic shock 12 years after mitral valve repair in nonrheumatic regurgitation [41]. This rescue technique permitted improved hemodynamics, rehabilitation, and eventual definitive management via surgical reoperation. Though not a definitive means to correct the pathology, it may be considered in cases of hemodynamic collapse requiring mechanical circulatory support until definitive treatment, surgical or percutaneous replacement, can be offered.
Challenges and Complications
Despite advancements in percutaneous procedures, MitraClip and transcatheter mitral valve replacement may impose challenges and complications. Because use of these devices in the failed mitral annuloplasty population is still “off-label,” case reports and limited case series will help shed light on potential limitations and yet to be seen complications. Complications of the MitraClip for FDA-approved use include single leaflet detachment, mitral stenosis, device embolization, among other common periprocedural complications such as bleeding, reintervention, and death. In a 3-year retrospective MitraClip analysis of 282 patients, the incidence of major bleeding was reported to be 7.4%, pericardial tamponade 1.9%, with in-hospital death, stroke, or myocardial infarction rates of 2.2%, 0.9%, and 0%, respectively [42]. In the 5-year follow-up from the EVEREST-II (Endovascular Valve Edge-to-Edge Repair Study) analysis, of 154 patients in the percutaneous repair cohort, single leaflet detachment was identified in nine patients within the first year and one additional patient 14 months out [43]. All patients subsequently underwent surgical repair or replacement. One patient had mitral stenosis (mitral valve area < 1.5 cm2) within a year, with no further reports at the 5-year mark. In this study, no device embolization was reported. Isolated case reports of mitral stenosis after MitraClip are limited and all required surgical mitral valve replacement [44,45,46]. Risk of subsequent stenosis has also been reported in a patient with end-stage renal disease and systemic lupus erythematosus, suggesting chronic inflammation and calcific disease may predispose stenotic lesions, similar to natural history valve degeneration in these populations [47, 48].
As previously mentioned, LVOT obstruction is a potential cause for concern when considering transcatheter mitral valve replacement. Though we have ways to mitigate risks with improved planning and combined procedures, such as the LAMPOON, other challenges and complications present during or after transcatheter replacement. Significant bleeding (5.6–8.0%), left ventricular perforation (0.4–6%), valve embolization (1.6–6%), left ventricular outflow tract obstruction (3.2–4%), emergent open cardiac surgery (2.0–8.0%), paravalvular leak, prosthetic valve thrombosis (2.0–3.6%), and death (1.2–6.0%) have all been described in the valve-in-valve or valve-in-ring patient populations [28, 49,50,51,52,53]. Eleid et al. reported early outcomes after transseptal valve-in-ring, and valve-in-valve, and severe mitral annular calcification valve replacements [54]. The valve-in-ring group (fifteen patients) had two cases of valve migration into the left atrium necessitating open surgical retrieval and replacement. Both patients had previous incomplete rings, again suggesting inadequate scaffolding for valve deployment and stabilization. Other notable findings in this limited cohort included 46.6% with incidental left ventricular pseudoaneurysm necessitating percutaneous closure, 13% with vascular access site bleeding requiring transfusions, and 60% met criteria for mitral stenosis (mean gradient > 5 mmHg or valve area < 1.5cm2). As challenges and complications are reported in the literature, more consideration and caution can be taken to properly plan percutaneous repair or replacement.
Conclusion
The decision for reoperation versus percutaneous interventions after annuloplasty failure is complex, especially as minimally invasive percutaneous techniques are still under development and currently used devices are “off-label.” Currently surgical reoperation remains the standard of care. However, as percutaneous options evolve and technology advances, percutaneous techniques will likely be the intervention of choice versus reoperation for many patients. In patients with high or prohibitive surgical risk, a multidisciplinary heart valve team approach is crucial for patient selection, periprocedural imaging, risk mitigation, procedural planning, and optimizing outcomes.
References
Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373(9672):1382–94.
American College of Cardiology/American Heart Association Task Force on Practice G, Society of Cardiovascular A, Society for Cardiovascular A, Interventions, Society of Thoracic S, Bonow RO, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114(5):e84–231.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521–643.
Schubert SA, Mehaffey JH, Charles EJ, Kron IL. Mitral valve repair: the French correction versus the American correction. Surg Clin North Am. 2017;97(4):867–88.
Cosgrove DM 3rd, Arcidi JM, Rodriguez L, Stewart WJ, Powell K, Thomas JD. Initial experience with the Cosgrove-Edwards Annuloplasty System. Ann Thorac Surg. 1995;60(3):499–503 discussion -4.
O’Gara PT, Grayburn PA, Badhwar V, Afonso LC, Carroll JD, Elmariah S, et al. 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(19):2421–49.
Badhwar V, Rankin JS, He X, Jacobs JP, Gammie JS, Furnary AP, et al. The Society of Thoracic Surgeons Mitral Repair/Replacement Composite Score: a report of the Society of Thoracic Surgeons Quality Measurement Task Force. Ann Thorac Surg. 2016;101(6):2265–71.
Goldstone AB, Cohen JE, Howard JL, Edwards BB, Acker AL, Hiesinger W, et al. A “Repair-All” strategy for degenerative mitral valve disease safely minimizes unnecessary replacement. Ann Thorac Surg. 2015;99(6):1983–90 discussion 90-1.
McGee EC, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128(6):916–24.
Alfieri O, Lapenna E. Systolic anterior motion after mitral valve repair: where do we stand in 2015? Eur J Cardiothorac Surg. 2015;48(3):344–6.
Brown ML, Abel MD, Click RL, Morford RG, Dearani JA, Sundt TM, et al. Systolic anterior motion after mitral valve repair: is surgical intervention necessary? J Thorac Cardiovasc Surg. 2007;133(1):136–43.
Kehl DW, Rader F, Pollick C, Trento A, Siegel RJ. Medical management (beta blocker +/- disopyramide) of left ventricular outflow gradient secondary to systolic anterior motion of the mitral valve after repair. Am J Cardiol. 2016;118(7):1053–6.
Flameng W, Meuris B, Herijgers P, Herregods MC. Durability of mitral valve repair in Barlow disease versus fibroelastic deficiency. J Thorac Cardiovasc Surg. 2008;135(2):274–82.
Ciarka A, Braun J, Delgado V, Versteegh M, Boersma E, Klautz R, et al. Predictors of mitral regurgitation recurrence in patients with heart failure undergoing mitral valve annuloplasty. Am J Cardiol. 2010;106(3):395–401.
Braun J, van de Veire NR, Klautz RJ, Versteegh MI, Holman ER, Westenberg JJ, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg. 2008;85(2):430–6 discussion 6-7.
El-Eshmawi A, Sun E, Boateng P, Pandis D, Rimsukcharoenchai C, Anyanwu A, et al. Lessons from reoperations for mitral stenosis after mitral valve repair. J Thorac Cardiovasc Surg. 2021;161(3):937–46.
Balsam LB, Grossi EA, Greenhouse DG, Ursomanno P, Deanda A, Ribakove GH, et al. Reoperative valve surgery in the elderly: predictors of risk and long-term survival. Ann Thorac Surg. 2010;90(4):1195–200 discussion 201.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72–e227.
Aphram G, De Kerchove L, Mastrobuoni S, Navarra E, Solari S, Tamer S, et al. Re-repair of the failed mitral valve: insights into aetiology and surgical management. Eur J Cardiothorac Surg. 2018;54(4):774–80.
Kilic A, Helmers MR, Han JJ, Kanade R, Acker MA, Hargrove WC, et al. Redo mitral valve surgery following prior mitral valve repair. J Card Surg. 2018;33(12):772–7.
Arnold SV, Chinnakondepalli KM, Spertus JA, Magnuson EA, Baron SJ, Kar S, et al. Health status after transcatheter mitral-valve repair in heart failure and secondary mitral regurgitation: COAPT trial. J Am Coll Cardiol. 2019;73(17):2123–32.
Sengupta A, Yazdchi F, Alexis SL, Percy E, Premkumar A, Hirji S, et al. Reoperative mitral surgery versus transcatheter mitral valve replacement: a systematic review. J Am Heart Assoc. 2021;10(6):e019854.
Grasso C, Ohno Y, Attizzani GF, Cannata S, Imme S, Barbanti M, et al. Percutaneous mitral valve repair with the MitraClip system for severe mitral regurgitation in patients with surgical mitral valve repair failure. J Am Coll Cardiol. 2014;63(8):836–8.
Agricola E, Taramasso M, Marini C, Montorfano M, Godino C, Alfieri O, et al. First-in-man MitraClip implantation to treat late postoperative systolic anterior motion: rare cause of tardive mitral repair failure. Circ Cardiovasc Interv. 2014;7(6):860–2.
Hanson ID, Hanzel GS, Shannon FL. Mitral valve repair after annuloplasty ring dehiscence using MitraClip. Catheter Cardiovasc Interv. 2016;88(2):301–6.
Ostovar R, Kuehnel RU, Erb M, Hartrumpf M, Claus T, Haase R, et al. How do transcatheter heart valves fit in mitral annuloplasty rings and which combination can be recommended? Thorac Cardiovasc Surg. 2019;67(4):257–65.
Hachinohe D, Latib A, Montorfano M, Colombo A. Transcatheter mitral valve implantation in rigid mitral annuloplasty rings: potential differences between complete and incomplete rings. Catheter Cardiovasc Interv. 2019;93(1):E71–E4.
Yoon SH, Whisenant BK, Bleiziffer S, Delgado V, Schofer N, Eschenbach L, et al. Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol. 2017;70(9):1121–31.
Bapat V. Valve-in-valve apps: why and how they were developed and how to use them. EuroIntervention. 2014;10(Suppl U):U44–51.
de Weger A, Ewe SH, Delgado V, Bax JJ. First-in-man implantation of a trans-catheter aortic valve in a mitral annuloplasty ring: novel treatment modality for failed mitral valve repair. Eur J Cardiothorac Surg. 2011;39(6):1054–6.
Hu J, Chen Y, Cheng S, Zhang S, Wu K, Wang W, et al. Transcatheter mitral valve implantation for degenerated mitral bioprostheses or failed surgical annuloplasty rings: a systematic review and meta-analysis. J Card Surg. 2018;33(9):508–19.
Meyer CG, Frick M, Lotfi S, Altiok E, Koos R, Kirschfink A, et al. Regional left ventricular function after transapical vs. transfemoral transcatheter aortic valve implantation analysed by cardiac magnetic resonance feature tracking. Eur Heart J Cardiovasc Imaging. 2014;15(10):1168–76.
Blanke P, Naoum C, Dvir D, Bapat V, Ong K, Muller D, et al. Predicting LVOT obstruction in transcatheter mitral valve implantation: concept of the neo-LVOT. JACC Cardiovasc Imaging. 2017;10(4):482–5.
Wang DD, Eng MH, Greenbaum AB, Myers E, Forbes M, Karabon P, et al. Validating a prediction modeling tool for left ventricular outflow tract (LVOT) obstruction after transcatheter mitral valve replacement (TMVR). Catheter Cardiovasc Interv. 2018;92(2):379–87.
Babaliaros VC, Greenbaum AB, Khan JM, Rogers T, Wang DD, Eng MH, et al. Intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction during transcatheter mitral valve replacement: first-in-human experience. JACC Cardiovasc Interv. 2017;10(8):798–809.
Khan JM, Babaliaros VC, Greenbaum AB, Foerst JR, Yazdani S, McCabe JM, et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol. 2019;73(20):2521–34.
Nunes Ferreira-Neto A, Dagenais F, Bernier M, Dumont E, Freitas-Ferraz AB, Rodes-Cabau J. Transcatheter mitral valve replacement with a new supra-annular valve: first-in-human experience with the altavalve system. JACC Cardiovasc Interv. 2019;12(2):208–9.
Bapat V, Rajagopal V, Meduri C, Farivar RS, Walton A, Duffy SJ, et al. Early experience with new transcatheter mitral valve replacement. J Am Coll Cardiol. 2018;71(1):12–21.
Kim JH, Lee SH, Joo HC, Youn YN, Yoo KJ, Chang BC, et al. Long-term clinical impacts of functional mitral stenosis after mitral valve repair. Ann Thorac Surg. 2021;111(4):1207–15.
Magne J, Senechal M, Mathieu P, Dumesnil JG, Dagenais F, Pibarot P. Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis. J Am Coll Cardiol. 2008;51(17):1692–701.
Salenger R, Diao X, Dawood MY, Herr DL, Sample GA, Pichard A, et al. Percutaneous rescue for critical mitral stenosis late after mitral valve repair. Ann Thorac Surg. 2016;102(5):e417–e8.
Eggebrecht H, Schelle S, Puls M, Plicht B, von Bardeleben RS, Butter C, et al. Risk and outcomes of complications during and after MitraClip implantation: experience in 828 patients from the German TRAnscatheter mitral valve interventions (TRAMI) registry. Catheter Cardiovasc Interv. 2015;86(4):728–35.
Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol. 2015;66(25):2844–54.
Cockburn J, Fragkou P, Hildick-Smith D. Development of mitral stenosis after single MitraClip insertion for severe mitral regurgitation. Catheter Cardiovasc Interv. 2014;83(2):297–302.
Osswald A, Al Jabbari O, Abu Saleh WK, Barker C, Ruhparwar A, Karmonik C, et al. Development of a severe mitral valve stenosis secondary to the treatment of mitral regurgitation with a single MitraClip. J Card Surg. 2016;31(3):153–5.
Singh K, Raphael J, Colquhoun D. A rare case of mitral stenosis after MitraClip placement: transesophageal echocardiography findings and examination. Anesth Analg. 2013;117(4):777–9 discussion 9.
Pope NH, Lim S, Ailawadi G. Late calcific mitral stenosis after MitraClip procedure in a dialysis-dependent patient. Ann Thorac Surg. 2013;95(5):e113–4.
Saji M, Ailawadi G, Fowler DE, LaPar DJ, Dent JM, Ragosta M, et al. Progressive mitral stenosis after MitraClip implantation in a patient with systemic inflammatory disease. Ann Thorac Surg. 2016;102(2):e89–91.
Eleid MF, Cabalka AK, Williams MR, Whisenant BK, Alli OO, Fam N, et al. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv. 2016;9(11):1161–74.
Urena M, Himbert D, Brochet E, Carrasco JL, Iung B, Nataf P, et al. Transseptal transcatheter mitral valve replacement using balloon-expandable transcatheter heart valves: a step-by-step approach. JACC Cardiovasc Interv. 2017;10(19):1905–19.
Bapat V, Pirone F, Kapetanakis S, Rajani R, Niederer S. Factors influencing left ventricular outflow tract obstruction following a mitral valve-in-valve or valve-in-ring procedure, part 1. Catheter Cardiovasc Interv. 2015;86(4):747–60.
Frisoli TM, Wang DD, Eng M, O’Neill WW, Paone G, Greenbaum AB. Mitral annuloplasty ring fracture and annular injury during transcatheter mitral valve-in-ring intervention. JACC Cardiovasc Interv. 2017;10(19):e181–e4.
Bapat VV, Khaliel F, Ihleberg L. Delayed migration of Sapien valve following a transcatheter mitral valve-in-valve implantation. Catheter Cardiovasc Interv. 2014;83(1):E150–4.
Eleid MF, Whisenant BK, Cabalka AK, Williams MR, Nejjari M, Attias D, et al. Early outcomes of percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv. 2017;10(19):1932–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Joseph reports no conflict of interest.
Dr. Nyman reports he receives education honoraria from Edwards Lifesciences, and in the past owned common stock in Edwards Lifesciences.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Structural Heart Disease
Rights and permissions
About this article
Cite this article
Joseph, K.M., Nyman, C. Mitral Valve Annuloplasty Failure and Percutaneous Treatment Options. Curr Cardiol Rep 23, 140 (2021). https://doi.org/10.1007/s11886-021-01574-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01574-4