Abstract
Among the long list of age-related complications, Alzheimer’s disease (AD) has the most dreadful impact on the quality of life due to its devastating effects on memory and cognitive abilities. Although a plausible correlation between the phosphatidylinositol 3-kinase (PI3K) signaling and different processes involved in neurodegeneration has been evidenced, few articles reviewed the task. The current review aims to unravel the mechanisms by which the PI3K pathway plays pro-survival roles in normal conditions, and also to discuss the original data obtained from international research laboratories on this topic. Responses to questions on how alterations of the PI3K/Akt signaling pathway affect Tau phosphorylation and the amyloid cascade are given. In addition, we provide a general overview of the association between oxidative stress, neuroinflammation, alterations of insulin signaling, and altered autophagy with aberrant activation of this axis in the AD brain. The last section provides a special focus on the therapeutic possibility of the PI3K/Akt/mTOR modulators, either categorized as chemicals or herbals, in AD. In conclusion, determining the correct timing for the administration of the drugs seems to be one of the most important factors in the success of these agents. Also, the role of the PI3K/Akt signaling axis in the progression or repression of AD widely depends on the context of the cells; generally speaking, while PI3K/Akt activation in neurons and neural stem cells is favorable, its activation in microglia cells may be harmful.




Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- ADDLs:
-
Aβ-derived diffusible ligands
- APP:
-
Amyloid precursor protein
- ARE:
-
Antioxidant response elements
- Aβ:
-
Amyloid β
- BDNF:
-
Brain-derived neurotrophic factor
- CaMKII:
-
Ca2+/calmodulin-dependent kinase II
- CK2:
-
Casein kinase 2
- CNS:
-
Central nervous system
- COX-1:
-
Cyclooxygenase-1
- COX-2:
-
Cyclooxygenase-2
- CXCL12:
-
C-X-C motif chemokine 12
- DNA:
-
Deoxyribonucleic acid
- eIF2-α:
-
Eukaryotic translation initiation factor-alpha
- FGF:
-
Fibroblast growth factor
- GABA:
-
γ-Aminobutyric acid
- GSK-3β:
-
Glycogen synthase kinase-3β
- HMK:
-
Halomethylketones 6
- IGF1:
-
Insulin-like growth factor-1
- IL1-β:
-
Interleukin 1 beta
- iNOS:
-
Inducible nitric oxide synthase
- IRS:
-
Insulin receptor substrate
- LTP:
-
Long-term potentiation
- mTOR:
-
Mammalian target of rapamycin
- NFTs:
-
Neurofibrillary tangles
- NF-κB:
-
Nuclear factor kappa light chain enhancer of activated B cells
- NMDA:
-
N-methyl-D-aspartate
- Nrf2:
-
Nuclear factor erythroid 2
- NRG1:
-
Neuregulin-1
- NSCs:
-
Neurons and neural stem cells
- PHF/SF:
-
Paired helical/straight filaments
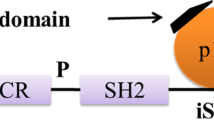
- PI3K:
-
Phosphatidyl inositol 3-kinase
- PIP2:
-
Phosphotidylinositol 4,5-Bisphosphate
- PIP3:
-
Phosphatidylinositol (3,4,5)-trisphosphate
- PTEN:
-
Phosphatase and tensin homolog
- PTMs:
-
Post-translational modifications
- ROS:
-
Reactive oxygen specious
- SH2:
-
Src homology 2
- SODs:
-
Superoxide dismutase
- TDZD:
-
Thiadiazolidinones 5
- TGF-β:
-
Transforming growth factor β
- TLR4:
-
Toll-like receptor 4
- TNF-α:
-
Tumor necrosis factor alpha
References
Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120(3):885–890
Haass C et al (1992) Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359(6393):322–325
Cheng Y et al (2013) Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev 65(4):1162–1197
Gong Y et al (2003) Alzheimer’s disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci 100(18):10417–10422
Karran E, Mercken M, De Strooper B (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discovery 10(9):698–712
Busche MA, Hyman BT (2020) Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci 23(10):1183–1193
Mietelska-Porowska A et al (2014) Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci 15(3):4671–4713
Hanger DP, Anderton BH, Noble W (2009) Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med 15(3):112–119
Noble W et al (2013) The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol 4:83
Rego AC, Oliveira CR (2003) Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res 28(10):1563–1574
Mosley RL et al (2006) Neuroinflammation, oxidative stress, and the pathogenesis of Parkinson’s disease. Clin Neurosci Res 6(5):261–281
Liu, Z., et al., Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxidative medicine and cellular longevity, 2017. 2017.
Swomley AM et al (1842) 2014 Abeta, oxidative stress in Alzheimer disease: evidence based on proteomics studies. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 8(1248):1257
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press, USA
Aseervatham GSB et al (2013) Environmental factors and unhealthy lifestyle influence oxidative stress in humans—an overview. Environ Sci Pollut Res 20(7):4356–4369
Nizzari, M., et al., Neurodegeneration in Alzheimer disease: role of amyloid precursor protein and presenilin 1 intracellular signaling. Journal of Toxicology, 2012. 2012.
Butterfield DA, Di Domenico F, Barone E (2014) Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochimica et Biophysica Acta BBA Molecular Basis of Disease 1842(9):1693–1706
Parsons MP, Raymond LA (2014) Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 82(2):279–293
Nakamura T, Lipton SA (2010) Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: possible pharmacological strategies. Cell Calcium 47(2):190–197
Nakamura T, Lipton S (2011) Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases. Cell Death Differ 18(9):1478–1486
Ferreiro E, Oliveira CR, Pereira CM (2008) The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol Dis 30(3):331–342
Hawking, Z.L., Alzheimer’s disease: the role of mitochondrial dysfunction and potential new therapies. Bioscience Horizons: The International Journal of Student Research, 2016. 9.
Picone, P., et al., Mitochondrial dysfunction: different routes to Alzheimer’s disease therapy. Oxidative medicine and cellular longevity, 2014. 2014.
Mutisya EM, Bowling AC, Beal MF (1994) Cortical cytochrome oxidase activity is reduced in Alzheimer’s disease. J Neurochem 63(6):2179–2184
Cassidy L et al (2020) Oxidative stress in alzheimer’s disease: a review on emergent natural polyphenolic therapeutics. Complement Ther Med 49:102294
Wong A et al (2001) Advanced glycation endproducts co-localize with inducible nitric oxide synthase in Alzheimer’s disease. Brain Res 920(1–2):32–40
Bradley-Whitman MA, Lovell MA (2015) Biomarkers of lipid peroxidation in Alzheimer disease (AD): an update. Arch Toxicol 89(7):1035–1044
Gella A, Durany N (2009) Oxidative stress in Alzheimer disease. Cell Adh Migr 3(1):88–93
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. Journal of Alzheimer’s Disease 57(4):1105–1121
Mattson MP, Chan SL (2003) Neuronal and glial calcium signaling in Alzheimer’s disease. Cell Calcium 34(4–5):385–397
Tamagno E et al (2003) H2O2 and 4-hydroxynonenal mediate amyloid β-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp Neurol 180(2):144–155
Butterfield DA, Boyd-Kimball D (2018) Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s disease. J Alzheimer’s Dis 62(3):1345–1367
Guglielmotto, M., et al., AGEs/RAGE complex upregulates BACE1 via NF-κB pathway activation. Neurobiology of aging, 2012. 33(1): p. 196. e13–196. e27.
Tamagno E et al (2012) Amyloid-β production: major link between oxidative stress and BACE1. Neurotox Res 22(3):208–219
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20(3):148–160
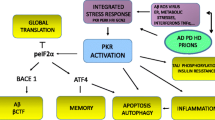
Ahmad F et al (2017) Reactive oxygen species-mediated loss of synaptic Akt1 signaling leads to deficient activity-dependent protein translation early in Alzheimer’s disease. Antioxid Redox Signal 27(16):1269–1280
O’Neill C (2013) PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol 48(7):647–653
Ye L et al (2019) FGF21 promotes functional recovery after hypoxic-ischemic brain injury in neonatal rats by activating the PI3K/Akt signaling pathway via FGFR1/β-klotho. Exp Neurol 317:34–50
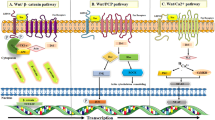
Vanhaesebroeck B et al (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11(5):329–341
Jaworski J et al (2005) Control of dendritic arborization by the phosphoinositide-3′-kinase–Akt–mammalian target of rapamycin pathway. J Neurosci 25(49):11300–11312
Kumar V et al (2005) Regulation of dendritic morphogenesis by Ras–PI3K–Akt–mTOR and Ras–MAPK signaling pathways. J Neurosci 25(49):11288–11299
Tahirovic S, Bradke F (2009) Neuronal polarity Cold Spring Harbor perspectives in biology 1(3):a001644
Hoyer S (2002) The aging brain Changes in the neuronal insulin insulin/receptor signal transduction cascade trigger late-onset sporadic Alzheimer disease (SAD) A mini review. J Neural transm 109(78):9911002
Zhao W et al (1999) Brain insulin receptors and spatial memory correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem 274(49):34893–34902
Wang Q et al (2003) Control of synaptic strength, a novel function of Akt. Neuron 38(6):915–928
Je HS et al (2011) Presynaptic protein synthesis required for NT-3-induced long-term synaptic modulation. Mol Brain 4(1):1
Je H-S et al (2009) Chemically inducible inactivation of protein synthesis in genetically targeted neurons. J Neurosci 29(21):6761–6766
Horwood JM et al (2006) Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci 23(12):3375–3384
Sato A et al (2010) Regulation of neural stem/progenitor cell maintenance by PI3K and mTOR. Neurosci Lett 470(2):115–120
Xu F et al (2020) Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 10:1–12
Bhattacharya S, Ray R, Johnson L (2005) Decreased apoptosis in polyamine depleted IEC-6 cells depends on Akt-mediated NF-κB activation but not GSK3β activity. Apoptosis 10(4):759–776
Ragot K et al (2011) α-Tocopherol impairs 7-ketocholesterol-induced caspase-3-dependent apoptosis involving GSK-3 activation and Mcl-1 degradation on 158N murine oligodendrocytes. Chem Phys Lipid 164(6):469–478
Su H-C et al (2012) Glycogen synthase kinase-3β regulates anti-inflammatory property of fluoxetine. Int Immunopharmacol 14(2):150–156
Watanabe S et al (2006) Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 25(19):2697–2707
Li B-S et al (2001) Activation of phosphatidylinositol-3 kinase (PI-3K) and extracellular regulated kinases (Erk1/2) is involved in muscarinic receptor-mediated DNA synthesis in neural progenitor cells. J Neurosci 21(5):1569–1579
Clarke DL et al (2000) Generalized potential of adult neural stem cells. Science 288(5471):1660–1663
Peltier J, O’Neill A, Schaffer DV (2007) PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol 67(10):1348–1361
Koh S-H, Lo EH (2015) The role of the PI3K pathway in the regeneration of the damaged brain by neural stem cells after cerebral infarction. J Clin Neurol 11(4):297–304
Gambarotta G et al (2004a) ErbB4 expression in neural progenitor cells (ST14A) is necessary to mediate neuregulin-1β1-induced migration. J Biol Chem 279(47):48808–48816
Gambarotta G et al (2004b) ErbB4 expression in neural progenitor cells (ST14A) is necessary to mediate neuregulin-1beta1-induced migration. J Biol Chem 279(47):48808–48816
Groszer M et al (2001) Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294(5549):2186–2189
Li J et al (1998) The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Can Res 58(24):5667–5672
Sun H et al (1999) PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3, 4, 5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci 96(11):6199–6204
Shi G et al (2011) PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun 404(4):941–945
Choi, H. and S.-H. Koh, Interaction between amyloid beta toxicity and the PI3K pathway in Alzheimer’s disease. Journal of Alzheimer’s Disease, 2016. 6(5).
Ren Z et al (2019) Astrocytic α7 nicotinic receptor activation inhibits amyloid-β aggregation by upregulating endogenous αB-crystallin through the PI3K/Akt signaling pathway. Curr Alzheimer Res 16(1):39–48
Ryu J et al (2016) Neuregulin-1 attenuates cognitive function impairments in a transgenic mouse model of Alzheimer’s disease. Cell Death Dis 7(2):e2117–e2117
Ariga T, Donald MP, Robert KY (2008) Thematic Review Series Sphingolipids. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease a review. J Lipid Res 49(6):1157–1175
O’Neill, C., et al., Insulin and IGF-1 signalling: longevity, protein homoeostasis and Alzheimer’s disease. 2012, Portland Press Ltd.
Koh S-H, Noh MY, Kim SH (2008) Amyloid-beta-induced neurotoxicity is reduced by inhibition of glycogen synthase kinase-3. Brain Res 1188:254–262
De Felice FG et al (2008) Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol Aging 29(9):1334–1347
Van Der Heide LP et al (2005) Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem 94(4):1158–1166
Chiu S-L, Chen C-M, Cline HT (2008) Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58(5):708–719
Lee C-C, Huang C-C, Hsu K-S (2011) Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology 61(4):867–879
Baker LD et al (2011) Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 68(1):51–57
Willette AA et al (2015) Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimer’s Dement 11(5):504-510. e1
Biessels GJ et al (2006) Risk of dementia in diabetes mellitus: a systematic review. The Lancet Neurology 5(1):64–74
Copps K, White M (2012) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55(10):2565–2582
Liu Y et al (2011) Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol 225(1):54–62
Hong M et al (1997) Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem 272(40):25326–25332
Park CR et al (2000) Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav 68(4):509–514
Zhao WQ et al (2008) Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J 22(1):246–260
Decker H et al (2010) Amyloid-β peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3β in primary cultured hippocampal neurons. J Neurosci 30(27):9166–9171
De Felice FG et al (2009) Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Aβ oligomers. Proc Natl Acad Sci 106(6):1971–1976
Caballero, B., et al., Interplay of pathogenic forms of human tau with different autophagic pathways. Aging cell, 2018. 17(1): p. e12692.
Wang C et al (2014a) Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J Pharmacol 740:312–320
Caccamo A et al (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-β, and tau effects on cognitive impairments. J Biol Chem 285(17):13107–13120
Annunziata I et al (2013) Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nat Commun 4(1):1–12
Ma T et al (2010) Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PloS one 5(9):e12845
Majd S, Power JH (2018) Oxidative stress and decreased mitochondrial superoxide dismutase 2 and peroxiredoxins 1 and 4 based mechanism of concurrent activation of AMPK and mTOR in Alzheimer’s disease. Curr Alzheimer Res 15(8):764–776
Chen L et al (2011) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radical Biol Med 50(5):624–632
Fu SC et al (2020) Cr VI induces ROS-mediated mitochondrial dependent-apoptosis in neuronal cells via the activation of Akt/ERK/AMPK signaling pathway. Toxicology in Vitro 65:104795
Kim, K.C., et al., 7, 8-Dihydroxyflavone suppresses oxidative stress-induced base modification in DNA via induction of the repair enzyme 8-oxoguanine DNA glycosylase-1. BioMed research international, 2013. 2013.
Surh Y-J, Kundu JK, Na H-K (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74(13):1526–1539
Singh B, Bhat HK (2012) Superoxide dismutase 3 is induced by antioxidants, inhibits oxidative DNA damage and is associated with inhibition of estrogen-induced breast cancer. Carcinogenesis 33(12):2601–2610
Shafi O (2016) Inverse relationship between Alzheimer’s disease and cancer, and other factors contributing to Alzheimer’s disease: a systematic review. BMC Neurol 16(1):1–17
Zhang M et al (2019) Effects of PI3K/Akt signaling pathway on serum C-reactive protein, serum amyloid A and cognitive dysfunction in mice with Alzheimer’s disease. INT J CLIN EXP MED 12(12):13437–13445
Ge Q et al (2017) High salt diet impairs memory-related synaptic plasticity via increased oxidative stress and suppressed synaptic protein expression. Mol Nutr Food Res 61(10):1700134
Koren J III et al (2009) Chaperone signalling complexes in Alzheimer’s disease. J Cell Mol Med 13(4):619–630
Shammas SL et al (2011) Binding of the molecular chaperone αB-crystallin to Aβ amyloid fibrils inhibits fibril elongation. Biophys J 101(7):1681–1689
Wilhelmus MM et al (2006) Small heat shock proteins inhibit amyloid-β protein aggregation and cerebrovascular amyloid-β protein toxicity. Brain Res 1089(1):67–78
Raman B et al (2005) αB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid β-peptide and β2-microglobulin. Biochem J 392(3):573–581
Volovik Y et al (2014) Differential regulation of the heat shock factor 1 and DAF 16 by neuronal nhl 1 in the nematode C elegans. Cell reports 9(6):2192–2205
Ren Z et al (2020) PNU282987 inhibits amyloid-β aggregation by upregulating astrocytic endogenous αB-crystallin and HSP-70 via regulation of the α7AChR, PI3K/Akt/HSF-1 signaling axis. Mol Med Rep 22(1):201–208
Perry VH, Nicoll JA, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol 6(4):193
Swardfager W et al (2010) Tumor necrosis factor alpha. Biol Psychiatry 68:930–941
Wu, Z., et al., Nutrients, microglia aging, and brain aging. Oxidative medicine and cellular longevity, 2016. 2016.
Fang W et al (2017) Identification and activation of TLR4-mediated signalling pathways by alginate-derived guluronate oligosaccharide in RAW264. 7 macrophages. Scientific reports 7(1):1–13
Nakajima K, Kohsaka S (2001) Microglia: activation and their significance in the central nervous system. J Biochem 130(2):169–175
Haslund-Vinding J et al (2017) NADPH oxidases in oxidant production by microglia: activating receptors, pharmacology and association with disease. Br J Pharmacol 174(12):1733–1749
Janyou A et al (2017) Dihydrocapsaicin attenuates blood brain barrier and cerebral damage in focal cerebral ischemia/reperfusion via oxidative stress and inflammatory. Sci Rep 7(1):1–11
Lipton JO, Sahin M (2014) The neurology of mTOR. Neuron 84(2):275–291
Yu H-J, Koh S-H (2017) The role of PI3K/AKT pathway and its therapeutic possibility in Alzheimer’s disease. Hanyang Med Rev 37(1):18–24
Garza JC et al (2012) Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3β/β-catenin signaling. Mol Psychiatry 17(8):790–808
Wang D et al (2012) Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J Biosci 37(1):91–101
Bigalke, B., et al., Adipocytokines and CD34+ progenitor cells in Alzheimer’s disease. PloS one, 2011. 6(5): p. e20286.
Oomura Y et al (2006) Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides 27(11):2738–2749
Harvey J, Solovyova N, Irving A (2006) Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res 45(5):369–378
Duvall A, Gallicchio V (2017) Lithium treatment in clinical medicine: history, current status and future use. J Cell Sci Ther 8(270):2
Rametti A et al (2008) Lithium down-regulates tau in cultured cortical neurons: a possible mechanism of neuroprotection. Neurosci Lett 434(1):93–98
Zhong J et al (2006) Lithium protects ethanol-induced neuronal apoptosis. Biochem Biophys Res Commun 350(4):905–910
Forlenza OV et al (2012) Does lithium prevent Alzheimer’s disease? Drugs Aging 29(5):335–342
Qian Y et al (2012) Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem Int 60(8):759–767
Luo S et al (2012) Genistein inhibits Aβ 25–35–induced neurotoxicity in PC12 cells via PKC signaling pathway. Neurochem Res 37(12):2787–2794
Hwang S, Lim JW, Kim H (2017) Inhibitory effect of lycopene on amyloid-β-induced apoptosis in neuronal cells. Nutrients 9(8):883
Tsang CK et al (2007) Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discovery Today 12(3–4):112–124
Nazio F et al (2013) mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol 15(4):406–416
Wang C et al (2014b) Targeting the mTOR signaling network for Alzheimer’s disease therapy. Mol Neurobiol 49(1):120–135
Bhaskar PT, Hay N (2007) The two TORCs and AKT. Dev Cell 12(4):487–502
Sarbassov DD et al (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307(5712):1098–1101
Majumder, S., et al., Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PloS one, 2011. 6(9): p. e25416.
Dowling RJ et al (2010) Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochimica et Biophysica Acta BBA Proteins and Proteomics 1804(3):433–439
Benjamin D et al (2011) Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discovery 10(11):868–880
Zaytseva YY et al (2012) mTOR inhibitors in cancer therapy. Cancer Lett 319(1):1–7
Bellozi PMQ et al (2019) NVP-BEZ235 (Dactolisib) has protective effects in a transgenic mouse model of Alzheimer’s disease. Front Pharmacol 10:1345
Bellozi PM et al (2016) Neuroprotective effects of the anticancer drug NVP-BEZ235 (dactolisib) on amyloid-β 1–42 induced neurotoxicity and memory impairment. Sci Rep 6:25226
Chang W, Teng J (2015) β-asarone prevents Aβ25-35-induced inflammatory responses and autophagy in SH-SY5Y cells: down expression Beclin-1, LC3B and up expression Bcl-2. Int J Clin Exp Med 8(11):20658
Deng M et al (2016) β-asarone improves learning and memory and reduces Acetyl Cholinesterase and Beta-amyloid 42 levels in APP/PS1 transgenic mice by regulating Beclin-1-dependent autophagy. Brain Res 1652:188–194
Zeng Y et al (2015) Tripchlorolide improves cognitive deficits by reducing amyloid β and upregulating synapse-related proteins in a transgenic model of Alzheimer’s Disease. J Neurochem 133(1):38–52
Zhu Z et al (2013) Arctigenin effectively ameliorates memory impairment in Alzheimer’s disease model mice targeting both β-amyloid production and clearance. J Neurosci 33(32):13138–13149
Song HS, Jang S, Kang SC (2018) Bavachalcone from Cullen corylifolium induces apoptosis and autophagy in HepG2 cells. Phytomedicine 40:37–47
Umezawa K et al (2018) Therapeutic activity of plant-derived alkaloid conophylline on metabolic syndrome and neurodegenerative disease models. Hum Cell 31(2):95–101
Song X et al (2017) Silibinin ameliorates anxiety/depression-like behaviors in amyloid β-treated rats by upregulating BDNF/TrkB pathway and attenuating autophagy in hippocampus. Physiol Behav 179:487–493
Li LS et al (2017) Dendrobium nobile Lindl alkaloid, a novel autophagy inducer, protects against axonal degeneration induced by Aβ25-35 in hippocampus neurons in vitro. CNS Neurosci Ther 23(4):329–340
Bhat, R., S. Budd, and J.M. Lindquist, Inhibition of GSK-3 as therapeutic strategy in disease: efficacy of AR-A014418. 2006, Wiley Online Library. p. 243–255.
Martinez A, Perez DI (2008) GSK-3 inhibitors: a ray of hope for the treatment of Alzheimer’s disease? J Alzheimer’s Dis 15(2):181–191
Licht-Murava A et al (2016) A unique type of GSK-3 inhibitor brings new opportunities to the clinic. Science Signaling 9(454):ra110–ra110
Akhondzadeh S et al (2003) Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomized and placebo-controlled trial. J Clin Pharm Ther 28(1):53–59
Shen Y et al (2011) An active fraction of Achyranthes bidentata polypeptides prevents apoptosis induced by serum deprivation in SH-SY5Y cells through activation of PI3K/AKT/Gsk3β pathways. Neurochem Res 36(11):2186–2194
Durairajan SSK et al (2012) Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol Aging 33(12):2903–2919
Wang Y et al (2013) Puerarin stimulates proliferation and differentiation and protects against cell death in human osteoblastic MG-63 cells via ER-dependent MEK/ERK and PI3K/Akt activation. Phytomedicine 20(10):787–796
Bhat RV et al (2018) The conundrum of GSK3 inhibitors: is it the dawn of a new beginning? J Alzheimer’s Dis 64(s1):S547–S554
Jeung, I.C., et al., Melissa officinalis L. extracts protect human retinal pigment epithelial cells against oxidative stress-induced apoptosis. International Journal of Medical Sciences, 2016. 13(2): p. 139.
Zhang G et al (2008) Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J Ethnopharmacol 115(3):441–448
Zhou T et al (2005) Large-scale isolation and purification of geniposide from the fruit of Gardenia jasminoides Ellis by high-speed counter-current chromatography. J Chromatogr A 1100(1):76–80
Nie, X., et al., Anti-aging properties of Dendrobium nobile Lindl.: from molecular mechanisms to potential treatments. Journal of Ethnopharmacology, 2020: p. 112839.
Gasiorowski K et al (2011) Flavones from root of Scutellaria baicalensis Georgi: drugs of the future in neurodegeneration? CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 10(2):184–191
Chen C et al (2018) Harpagoside rescues the memory impairments in chronic cerebral hypoperfusion rats by inhibiting PTEN activity. J Alzheimer’s Dis 63(2):445–455
Uddin M, Kabir M, Kabir T (2019) Emerging signal regulating potential of genistein against Alzheimer disease: a promising molecule of interest. Front Cell Dev Biol 7:197
Xiong N et al (2011) Potential autophagy enhancers attenuate rotenone-induced toxicity in SH-SY5Y. Neurosci 199:292–302
Capiralla H et al (2012) Resveratrol mitigates lipopolysaccharide-and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J Neurochem 120(3):461–472
Fu J et al (2012) Hypolipidemic activity in Sprague-Dawley rats and constituents of a novel natural vegetable oil from Cornus wilsoniana fruits. J Food Sci 77(8):H160–H169
Hui Y et al (2018) Resveratrol attenuates the cytotoxicity induced by amyloid-β 1–42 in PC12 cells by upregulating heme oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem Res 43(2):297–305
Wang S et al (2018) Berberine alleviates tau hyperphosphorylation and axonopathy-associated with diabetic encephalopathy via restoring PI3K/Akt/GSK3β pathway. J Alzheimer’s Dis 65(4):1385–1400
Lee Y-R et al (2007) Peroxisome proliferator-activated receptor γ and retinoic acid receptor synergistically up-regulate the tumor suppressor PTEN in human promyeloid leukemia cells. Int J Hematol 85(3):231–237
Noh MY et al (2013) The early activation of PI3K strongly enhances the resistance of cortical neurons to hypoxic injury via the activation of downstream targets of the PI3K pathway and the normalization of the levels of PARP activity, ATP, and NAD+. Mol Neurobiol 47(2):757–769
Zhang L et al (2017) TRPML1 participates in the progression of Alzheimer’s disease by regulating the PPARγ/AMPK/Mtor signalling pathway. Cell Physiol Biochem 43(6):2446–2456
Mahadevan D et al (2012) Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer 48(18):3319–3327
Tramutola A, Lanzillotta C, Di Domenico F (2017) Targeting mTOR to reduce Alzheimer-related cognitive decline: from current hits to future therapies. Expert Rev Neurother 17(1):33–45
Silva MC et al (2020) Prolonged tau clearance and stress vulnerability rescue by pharmacological activation of autophagy in tauopathy neurons. Nat Commun 11(1):1–18
Drake, R., The importance and practical application of autophagy in human health.
Ahmed, A.R., et al., Directly imaging the localisation and photosensitization properties of the pan-mTOR inhibitor, AZD2014, in living cancer cells. Journal of Photochemistry and Photobiology B: Biology, 2020. 213: p. 112055.
Yu, Z., et al., Relationship between adiponectin gene polymorphisms and late-onset Alzheimer’s disease. PloS one, 2015. 10(4): p. e0125186.
Couvineau A et al (2019) Orexins as novel therapeutic targets in inflammatory and neurodegenerative diseases. Front Endocrinol 10:709
Zhao Y et al (2018) Melatonin protects against Aβ-induced neurotoxicity in primary neurons via miR-132/PTEN/AKT/FOXO3a pathway. BioFactors 44(6):609–618
Acknowledgements
The authors would like to express their gratitude to Shahid Beheshti University of Medical Sciences (Tehran, Iran) for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Razani, E., Pourbagheri-Sigaroodi, A., Safaroghli-Azar, A. et al. The PI3K/Akt signaling axis in Alzheimer’s disease: a valuable target to stimulate or suppress?. Cell Stress and Chaperones 26, 871–887 (2021). https://doi.org/10.1007/s12192-021-01231-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-021-01231-3