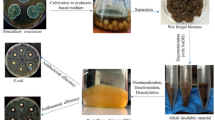
Abstract
Despite being widely available, Saccharomyces cerevisiae has not been widely explored for direct extraction of chitosan biopolymer for antimicrobial applications. In our study, S. cerevisiae from Baker’s yeast and Aspergillus niger from moldy onion extracts are studied as alternative sources of chitosan; and S cerevisiae chitosan tested for antimicrobial efficacy. The properties of S. cerevisiae chitosan are compared with moldy onion chitosan and shrimp chitosan extracted from shrimp shells. Chitosan extracted from S. cerevisiae is tested for antimicrobial efficacy against Staphylococcus Aureus. The maximum yields of fungal chitosan are 20.85 ± 0.35 mg/g dry S. cerevisiae biomass at 4th day using a culture broth containing sodium acetate, and 16.15 ± 0.95 mg/g dry A. niger biomass at 12th day. The degree of deacetylation (DD%) of the extracted fungal chitosan samples from S. cerevisiae and A. niger is found to be 63.4%, and 61.2% respectively, using Fourier Transform Infrared Spectroscopy. At a concentration of 2 g/L, S. cerevisiae chitosan shows the maximum inhibition zone diameter of 15.48 ± 0.07 mm. Baker’s yeast S cerevisiae biomass and A. niger from moldy onions has not been previously explored as a source of extractible fungal chitosan. This study gives insight that S. cerevisiae and A. niger from agricultural or industrial wastes could be a potential biomass source for production of the chitosan biopolymer. The S. cerevisiae chitosan displayed effective antimicrobial properties against S aureus, indicating the viablitiy of S cerevisae as a resource for extraction of high-quality chitosan.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, NI, upon reasonable request.
References
Muzzarelli, R. A. A. (2015). Chitin: Metal ion chelation and enzyme immobilization. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials, 11, 1493–1498. CRC Press.
Dash, M., Chiellini, F., Ottenbrite, R. M., & Chiellini, E. (2011, August 1). Chitosan - A versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science (Oxford). Elsevier Ltd. https://doi.org/10.1016/j.progpolymsci.2011.02.001
Namboodiri, M. M. T., & Pakshirajan, K. (2019). Sustainable and green approach of chitosan production from Penicillium citrinum biomass using industrial wastewater as a cheap substrate. Journal of environmental management, 240, 431–440.
Zhang, X., Hassanzadeh, P., Miyake, T., Jin, J., & Rolandi, M. (2016). Squid beak inspired water processable chitosan composites with tunable mechanical properties. Journal of Materials Chemistry B, 4(13), 2273–2279.
Rabea, E. I., Badawy, M.E.-T., Stevens, C. V., Smagghe, G., & Steurbaut, W. (2003). Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules, 4(6), 1457–1465.
Azuma, K., Ifuku, S., Osaki, T., Okamoto, Y., & Minami, S. (2014). Preparation and biomedical applications of chitin and chitosan nanofibers. Journal of biomedical nanotechnology, 10(10), 2891–2920.
Niederhofer, A., & Müller, B. W. (2004). A method for direct preparation of chitosan with low molecular weight from fungi. European Journal of Pharmaceutics and Biopharmaceutics, 57(1), 101–105.
No, H. K., & Meyers, S. P. (2000). Application of chitosan for treatment of wastewaters. In Reviews of environmental contamination and toxicology (pp. 1–27). Springer.
Mo, J., Yang, Q., Zhang, N., Zhang, W., Zheng, Y., & Zhang, Z. (2018). A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. Journal of environmental management, 227, 395–405.
Boardman, S. J., Lad, R., Green, D. C., & Thornton, P. D. (2017). Chitosan hydrogels for targeted dye and protein adsorption. Journal of Applied Polymer Science, 134(21).
Younes, I., & Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine drugs, 13(3), 1133–1174.
Nwe, N., Furuike, T., & Tamura, H. (2011). Chitosan from aquatic and terrestrial organisms and microorganisms: production, properties and applications. In BM Johnson and ZE Berkel (Ed.), Biodegradable Materials.
Philibert, T., Lee, B. H., & Fabien, N. (2017, April 1). Current status and new perspectives on chitin and chitosan as functional biopolymers. Applied Biochemistry and Biotechnology. Humana Press Inc. https://doi.org/10.1007/s12010-016-2286-2
Ghormade, V., Pathan, E. K., & Deshpande, M. V. (2017, November 1). Can fungi compete with marine sources for chitosan production? International Journal of Biological Macromolecules. Elsevier B.V. https://doi.org/10.1016/j.ijbiomac.2017.01.112
Elsoud, M. M. A., & Kady, E. M. El. (n.d.). Current trends in fungal biosynthesis of chitin and chitosan. https://doi.org/10.1186/s42269-019-0105-y
Pochanavanich, P., & Suntornsuk, W. (2002). Fungal chitosan production and its characterization. Letters in Applied Microbiology, 35(1), 17–21. https://doi.org/10.1046/j.1472-765X.2002.01118.x
AliIkram-ul-Haq, S., Qadeer, M. A., & Iqbal, J. (2002). Production of citric acid by Aspergillus niger using cane molasses in a stirred fermentor. Electronic Journal of Biotechnology, 5(3), 258–271. https://doi.org/10.2225/vol5-issue3-fulltext-3
Hahn, T., Paul, A., Falabella, P., Zibek, S., & Salvia, R. (2020). Current state of chitin puri fi cation and chitosan production from insects, (July). https://doi.org/10.1002/jctb.6533
Sebastian, J., Rouissi, T., & Brar, S. K. (2020). challenges. https://doi.org/10.1016/B978-0-12-817970-3.00014-6
Massoud, R., Hadiani, M. R., Hamzehlou, P., & Khosravi-Darani, K. (2019, January 1). Bioremediation of heavy metals in food industry: Application of Saccharomyces cerevisiae. Electronic Journal of Biotechnology. Pontificia Universidad Catolica de Valparaiso. https://doi.org/10.1016/j.ejbt.2018.11.003
Jacob, F. F., Hutzler, M., & Methner, F. J. (2019). Comparison of various industrially applicable disruption methods to produce yeast extract using spent yeast from top-fermenting beer production: Influence on amino acid and protein content. European Food Research and Technology, 245(1), 95–109. https://doi.org/10.1007/s00217-018-3143-z
Avramia, I., & Amariei, S. (2021). Spent Brewer’s yeast as a source of insoluble β-glucans. International Journal of Molecular Sciences, 22(2), 1–26. https://doi.org/10.3390/ijms22020825
Soares, E. V., & Soares, H. M. V. M. (2012, May 3). Bioremediation of industrial effluents containing heavy metals using brewing cells of Saccharomyces cerevisiae as a green technology: A review. Environmental Science and Pollution Research. Springer. https://doi.org/10.1007/s11356-011-0671-5
Brady, D., & Duncan, J. R. (1994). Bioaccumulation of metal cations by Saccharomyces cerevisiae. Applied Microbiology and Biotechnology, 41(1), 149–154. https://doi.org/10.1007/BF00166098
Bemena, L. D., Mukama, O., Neiman, A. M., Li, Z., Gao, X. D., & Nakanishi, H. (2017). In vitro reconstitution of the yeast spore wall dityrosine layer discloses the mechanism of its assembly. Journal of Biological Chemistry, 292(38), 15880–15891. https://doi.org/10.1074/jbc.M117.786202
Younis, G., Awad, A., Dawod, R. E., & Yousef, N. E. (2017). Antimicrobial activity of yeasts against some pathogenic bacteria. Veterinary World, 10(8), 979–983. https://doi.org/10.14202/vetworld.2017.979-983
Lesage, G., & Bussey, H. (2006). Cell Wall Assembly in Saccharomyces cerevisiae. Microbiology and Molecular Biology Reviews, 70(2), 317–343. https://doi.org/10.1128/mmbr.00038-05
Tong, Z., Zheng, X., Tong, Y., Shi, Y. C., & Sun, J. (2019, February 4). Systems metabolic engineering for citric acid production by Aspergillus niger in the post-genomic era. Microbial Cell Factories. BioMed Central Ltd. https://doi.org/10.1186/s12934-019-1064-6
Ma, Q., Gao, X., Bi, X., Xia, M., Han, Q., Peng, M., & Wang, M. (2021). Combination of steam explosion and ionic liquid pretreatments for efficient utilization of fungal chitin from citric acid fermentation residue. Biomass and Bioenergy, 145, 105967. https://doi.org/10.1016/j.biombioe.2021.105967
Palumbo, J. D., O’keeffe, T. L., & Abbas, H. K. (2008). Microbial interactions with mycotoxigenic fungi and mycotoxins. Toxin Reviews, 27(42067), 261–285. https://doi.org/10.1080/15569540802416301
Ghormade, V. (2017). Diversity of natural yeast flora of grapes and its significance in wine making diversity of natural yeast flora of grapes and its significance in wine making, (May). https://doi.org/10.1007/978-981-10-2621-8
Plascencia-Jatomea, M., Susana, M., Gómez, Y., & Velez-Haro, J. M. (2014). Aspergillus spp. (Black Mold). In Postharvest Decay: Control Strategies (pp. 267–286). Elsevier Inc. https://doi.org/10.1016/B978-0-12-411552-1.00008-9
Matica, M. A., Aachmann, F. L., Tøndervik, A., Sletta, H., & Ostafe, V. (2019, December 1). Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. International Journal of Molecular Sciences. MDPI AG. https://doi.org/10.3390/ijms20235889
Askarian, F., Sangvik, M., Hanssen, A. M., Snipen, L., Sollid, J. U. E., & Johannessen, M. (2014). Staphylococcus aureus nasal isolates from healthy individuals cause highly variable host cell responses in vitro: The Tromsø Staph and Skin Study. Pathogens and Disease, 70(2), 158–166. https://doi.org/10.1111/2049-632X.12099
Maghsoodi, V., & Yaghmaei, S. (2010). Comparison of solid substrate and submerged fermentation for chitosan production by aspergillus niger. Scientia Iranica, 17(2 C), 153–157.
McClenny, N. (2005). Laboratory detection and identification of Aspergillus species by microscopic observation and culture: The traditional approach. Medical Mycology, 43(s1), 125–128. https://doi.org/10.1080/13693780500052222
Maghsoodi, V., Razavi, J., & Yaghmaei, S. (2009). Production of chitosan by submerged fermentation from Aspergillus niger. Scientia Iranica, 16(2 C), 145–148. Retrieved from www.SID.ir
Shehu, K., & Bello, M. T. (2011). Effect of environmental factors on the growth of Aspergillus species associated with stored millet grains in Sokoto. Nigerian Journal of Basic and Applied Science, 19(2), 218–223. Retrieved from http://www.ajol.info/index.php/njbas/index
Kumaresapillai, N., Basha, R. A., & Sathish, R. (2011). Production and evaluation of chitosan from Aspergillus niger MTCC strains. Iranian Journal of Pharmaceutical Research, 10(3), 553–558. https://doi.org/10.22037/ijpr.2011.1003
Briza, P., Ellinger, A., Winkler, G., & Breitenbach, M. (1988). Chemical composition of the yeast ascospore wall. The second outer layer consists of chitosan. Journal of Biological Chemistry, 263(23), 11569–11574.
François, J. M. (2007). A simple method for quantitative determination of polysaccharides in fungal cell walls. Nature Protocols, 1(6), 2995–3000. https://doi.org/10.1038/nprot.2006.457
Abasian, L., Shafiei Alavijeh, R., Satari, B., & Karimi, K. (2020). Sustainable and effective chitosan production by dimorphic fungus Mucor rouxii via replacing yeast extract with fungal extract. Applied Biochemistry and Biotechnology, 191(2), 666–678. https://doi.org/10.1007/s12010-019-03220-w
Cabib, E., Blanco, N., Grau, C., Rodríguez-Peña, J. M., & Arroyo, J. (2007). Crh1p and Crh2p are required for the cross-linking of chitin to ?(1–6)glucan in the Saccharomyces cerevisiae cell wall. Molecular Microbiology, 63(3), 921–935. https://doi.org/10.1111/j.1365-2958.2006.05565.x
Maghsoodi, V., & Yaghmaei, S. (2010). Comparison of solid substrate and submerged fermentation for chitosan production by Aspergillus niger. Transactions C: Chemistry and Chemical Engineering, 17(2). Retrieved from www.SID.ir
Viccini, G., Mitchell, D. A., Gern, J. C., Krieger, N., Mitchell, D. A., Boit, S. D., … Von Meien, O. F. (2001). Analysis of growth kinetic profiles in solid-state fermentation solid-state fermentation bioreactors View project Special Issue in Recent advances in Biocatalysis and Biotransformations (Published in BBA-Proteins and Proteomics) View project Analysis of Growth Kinetic Profiles in Solid-State Fermentation. Food Technology and Biotechnology. Retrieved from https://www.researchgate.net/publication/224893510
Vaingankar, P. N., & Juvekar, A. R. (2014). Fermentative production of mycelial chitosan from Zy-gomycetes: Media optimization and physico-chemical characterization. Advances in Bioscience and Biotechnology, 5, 940. https://doi.org/10.4236/abb.2014.512108
Da Silva Amorim, R. V., De Souza, W., Fukushima, K., & De Campos-Takaki, G. M. (2001). Faster chitosan production by Mucoralean strains in submerged culture. Brazilian Journal of Microbiology, 32(1), 20–23. https://doi.org/10.1590/s1517-83822001000100005
Cardoso, A., Lins, C. I. M., Dos Santos, E. R., Silva, M. C. F., & Campos-Takaki, G. M. (2012). Microbial enhance of chitosan production by Rhizopus arrhizus using agroindustrial substrates. Molecules, 17(5), 4904–4914. https://doi.org/10.3390/molecules17054904
Cardoso, A., Marques, A., Campos Marinho, P. H., Shiosaki, R. K., & Campos Takaki, G. M. (2010). Influence of the heavy metals on chitosan production by Absidia corymbifera UCP 0134 (pp. 176–180). World Scientific Pub Co Pte Lt. https://doi.org/10.1142/9789814322119_0039
Maghsoudi, V., Yaghmaei, S., & Beygi, S. (2008). Influence of different nitrogen sources on amount of chitosan production by Aspergillus niger in solid state fermentation. Retrieved from https://www.sid.ir/en/journal/ViewPaper.aspx?id=109003
Nwe, N., Tetsuya, & Tamur, H. (2010). Production of fungal chitosan by enzymatic method and applications in plant tissue culture and tissue engineering: 11 years of our progress, Present Situation and Future Prospects. In Biopolymers. Sciyo. https://doi.org/10.5772/10261
Fazenda, M. L., Seviour, R., McNeil, B., & Harvey, L. M. (2008). Submerged culture fermentation of “Higher Fungi”: The macrofungi. Advances in Applied Microbiology, 63, 33–103. https://doi.org/10.1016/S0065-2164(07)00002-0
Ferreira, J. A., Mahboubi, A., Lennartsson, P. R., & Taherzadeh, M. J. (2016). Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresource Technology, 215, 334–345. https://doi.org/10.1016/j.biortech.2016.03.018
Zhang, H., Tachikawa, H., Gao, X. D., & Nakanishi, H. (2014). Applied usage of yeast spores as chitosan beads. Applied and Environmental Microbiology, 80(16), 5098–5105. https://doi.org/10.1128/AEM.00677-14
Monarul Islam, M., Md Masum, S., Mahbubur Rahman, M., Ashraful Islam Molla, M., Shaikh, A. A., & Roy, S. (2011). Preparation of chitosan from shrimp shell and investigation of its properties. International Journal of Basic & Applied Sciences IJBAS-IJENS, 11(01).
Hossain, M. S., & Iqbal, A. (2014). Production and characterization of chitosan from shrimp waste. J. Bangladesh Agril. Univ, 12(1), 153–160. Retrieved from https://www.banglajol.info/index.php/JBAU/article/view/21405
Tharanathan, R. N., & Kittur, F. S. (2003). Chitin - The Undisputed Biomolecule of Great Potential. Critical Reviews in Food Science and Nutrition. https://doi.org/10.1080/10408690390826455
Kumirska, J., Czerwicka, M., Kaczyński, Z., Bychowska, A., Brzozowski, K., Thöming, J., & Stepnowski, P. (2010). Application of spectroscopic methods for structural analysis of chitin and chitosan. Marine Drugs. MDPI AG. https://doi.org/10.3390/md8051567
Czechowska-Biskup, R., Jarosińska, D., Rokita, B., Ulański, P., & Rosiak, J. M. (2012). Determination of degree of deacetylation of chitosan-comparision of methods. Progress on Chemistry and Application of Chitin and its Derivatives, 17, 5–20.
Gregorio-Jauregui, K. M., Pineda, M. G., Rivera-Salinas, J. E., Hurtado, G., Saade, H., Martinez, J. L., Ilyina, A., & López, R. G. (2012). One-step method for preparation of magnetic nanoparticles coated with chitosan. Journal of Nanomaterials. https://doi.org/10.1155/2012/813958
Domszy, J., & Roberts, G. (1985). Evaluation of infrared spectroscopic techniques for analysing chitosan. Die Makromolekulare Chemie, 186(8), 1671–1677. https://doi.org/10.1002/macp.1985.021860815
Mozafari, M., Moztarzadeh, J., Alhosseini, N., & AsgariKargozars, D. (2012). Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. International Journal of Nanomedicine, 7, 25. https://doi.org/10.2147/ijn.s25376
Bhuvaneshwari, S., & Sivasubramanian, V. (2013). Comparative studies for chitosan yield and chelating ability of Aspergillus niger and Rhizopus oryzae. Indian Journal of Biotechnology (Vol. 12). Retrieved from http://nopr.niscair.res.in/handle/123456789/21852
Chatterjee, S., Adhya, M., Guha, A. K., & Chatterjee, B. P. (2005). Chitosan from Mucor rouxii: Production and physico-chemical characterization. Process Biochemistry, 40, 395–400. https://doi.org/10.1016/j.procbio.2004.01.025
Yang, L., Li, X., Lai, C., Fan, Y., Ouyang, J., & Yong, Q. (2017). Fungal chitosan production using xylose rich of corn stover prehydrolysate by Rhizopus oryzae. Biotechnology and Biotechnological Equipment, 31(6), 1160–1166. https://doi.org/10.1080/13102818.2017.1370678
Gharieb, M. M., Sabha, ;, El-Sabbagh, M., Shalaby, M. A., & Darwesh, O. M. (2015). Production of chitosan from different species of zygomycetes and its antimicrobial activity. International Journal of Scientific & Engineering Research, 6(4).
Kaya, M., Akata, I., Baran, T., & Menteş, A. (2015). Physicochemical properties of chitin and chitosan produced from medicinal fungus (Fomitopsis pinicola). Food Biophysics, 10(2), 162–168. https://doi.org/10.1007/s11483-014-9378-8
Goy, R. C., Morais, S. T. B., & Assis, O. B. G. (2016). Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. Coli and S. aureus growth. Brazilian Journal of Pharmacognosy, 26(1), 122–127. https://doi.org/10.1016/j.bjp.2015.09.010
Chang, A. K. T., Frias, R. R., Alvarez, L. V., Bigol, U. G., & Guzman, J. P. M. D. (2019). Comparative antibacterial activity of commercial chitosan and chitosan extracted from Auricularia sp. Biocatalysis and Agricultural Biotechnology, 17, 189–195. https://doi.org/10.1016/J.BCAB.2018.11.016
Shanmugam, A., Kathiresan, K., & Nayak, L. (2016). Preparation, characterization and antibacterial activity of chitosan and phosphorylated chitosan from cuttlebone of Sepia kobiensis (Hoyle, 1885). Biotechnology Reports, 9, 25–30.
Logesh, A. R., Thillaimaharani, K., Sharmila, K., Kalaiselvam, M., & Raffi SM. (2012). Production of chitosan from endolichenic fungi isolated from mangrove environment and its antagonistic activity. Asian Pacific Journal of Tropical Biomedicine, 2(2), 140–143. Retrieved from https://www.sciencedirect.com/science/article/pii/S2221169111602086
Nair StJoseph, S., Kayakkeel Assainar, S., Assainar, S. K., & Nair, S. P. (2014). Action of chitosan and its derivatives on clinical pathogens antifungal activity View project Antimicrobial activity View project Action of Chitosan and its derivatives on clinical pathogens. Int.J.Curr.Microbiol.App.Sci, 3(10), 748–759. Retrieved from http://www.ijcmas.com
Tayel, A. A., Moussa, S., Opwis, K., Knittel, D., Schollmeyer, E., & Nickisch-Hartfiel, A. (2010). Inhibition of microbial pathogens by fungal chitosan. International Journal of Biological Macromolecules, 47, 10–14. https://doi.org/10.1016/j.ijbiomac.2010.04.005
Tayel, A. A., Gharieb, M. M., Zaki, H. R., & Elguindy, N. M. (2016). Bio-clarification of water from heavy metals and microbial effluence using fungal chitosan. International Journal of Biological Macromolecules, 83, 277–281. Retrieved from https://www.sciencedirect.com/science/article/pii/S0141813015301689
Dutta, P. K., Tripathi, S., Mehrotra, G. K., & Dutta, J. (2009). Perspectives for chitosan based antimicrobial films in food applications. Food Chemistry, 114(4), 1173–1182.
Burkatovskaya, M., Tegos, G. P., Swietlik, E., Demidova, T. N., Castano, A. P., & Hamblin, M. R. (n.d.). Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials, 27(22), 4157–4164.
Liu, N., Chen, X. G., Park, H. J., Liu, C. G., Liu, C. S., Meng, X. H., & Yu, L. J. (2006). Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydrate Polymers, 64(1), 60–65. https://doi.org/10.1016/j.carbpol.2005.10.028
Qi, L., Xu, Z., Jiang, X., Hu, C., & Zou, X. (2004). Preparation and antibacterial activity of chitosan nanoparticles. Elsevier. https://doi.org/10.1016/j.carres.2004.09.007
Palermo, E. F., & Kuroda, K. (2010, August 19). Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Applied Microbiology and Biotechnology. Springer. https://doi.org/10.1007/s00253-010-2687-z
Gabriel, G. J., Maegerlein, J. A., Nelson, C. F., Dabkowski, J. M., Eren, T., Nüsslein, K., & Tew, G. N. (2009). Comparison of facially amphiphilic versus segregated monomers in the design of antibacterial copolymers. Chemistry - A European Journal, 15(2), 433–439. https://doi.org/10.1002/chem.200801233
de Freitas, R. A., Drenski, M. F., Alb, A. M., & Reed, W. F. (2010). Characterization of stability, aggregation, and equilibrium properties of modified natural products; The case of carboxymethylated chitosans. Materials Science and Engineering C, 30(1), 34–41. https://doi.org/10.1016/j.msec.2009.08.005
Hosseinnejad, M., & Mahdi, S. (2016). International Journal of Biological Macromolecules Evaluation of different factors affecting antimicrobial properties of chitosan. International Journal of Biological Macromolecules, 85, 467–475. https://doi.org/10.1016/j.ijbiomac.2016.01.022
Parapouli, M., Vasileiadis, A., Afendra, A. S., & Hatziloukas, E. (2020). Saccharomyces cerevisiae and its industrial applications. AIMS Press. https://doi.org/10.3934/microbiol.2020001
Muzzarelli, R. A. A., Tanfani, F., & Scarpini, G. (1980). Chelating, film-forming, and coagulating ability of the chitosan–glucan complex from Aspergillus niger industrial wastes. Biotechnology and Bioengineering, 22(4), 885–896. https://doi.org/10.1002/bit.260220412
Brasselet, C., Pierre, G., Dubessay, P., Dols-Lafargue, M., Coulon, J., Maupeu, J., Vallet-Courbin, A., de Baynast, H., Doco, T., Michaud, P., & Delattre, C. (2019). Modification of chitosan for the generation of functional derivatives. Applied Sciences, 9(7), 1321. https://doi.org/10.3390/app9071321
Harish Prashanth, K. V., & Tharanathan, R. N. (2007). Chitin/chitosan: Modifications and their unlimited application potential-an overview. Trends in Food Science and Technology, 18(3), 117–131. https://doi.org/10.1016/j.tifs.2006.10.022
Acknowledgements
The authors thank Mr. Mizanul Hoque and Md. Abu Sayeed Sheikh for assistance in carrying out the S cerevisiae-based fungal chitosan extraction in ammonium acetate media and for their assistance in carrying out the antimicrobial efficacy studies. The authors are thankful for technical support from the Department of Chemistry, Bangladesh University of Engineering Technology. We are grateful for the partial scholarship provided by the BUET Chemical Engineering Forum (BCEF) funding Md. Masirul Afroz.
Funding
This work was funded by the Committee for Advanced Studies and Research (CASR), Bangladesh, University of Engineering and Technology.
Author information
Authors and Affiliations
Contributions
Conceptualization: NI and MA; methodology: MA, PS, and NH; formal analysis and investigation: MA, PS, and NH; writing—original draft preparation: MA and NI; writing—review and editing: MA, PS, NH, and NI; funding acquisition: NI; resources: NI; supervision: NI.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of the results of this manuscript have been presented in the Fifth International Conference for Chemical Engineering, ICChE 2017.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Afroz, M.M., Kashem, M.N.H., Piash, K.M.P.S. et al. Saccharomyces Cerevisiae as an Untapped Source of Fungal Chitosan for Antimicrobial Action. Appl Biochem Biotechnol 193, 3765–3786 (2021). https://doi.org/10.1007/s12010-021-03639-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03639-0