Abstract—
Evodiamine, an alkaloid component in the fruit of Evodia, has been shown to have biological functions such as antioxidant and anti-inflammatory. But whether evodiamine plays an improvement role on mastitis has not been studied. To investigate the effect and mechanism of evodiamine on lipopolysaccharide (LPS)-induced mastitis was the purpose of this study. In animal experiments, the mouse mastitis model was established by injecting LPS into the canals of the mammary gland. The results showed that evodiamine could significantly relieve the pathological injury of breast tissue and the production of pro-inflammatory cytokines and inhibit the activation of inflammation-related pathways such as AKT, NF-κB p65, ERK1/2, p38, and JNK. In cell experiments, the mouse mammary epithelial cells (mMECs) were incubated with evodiamine for 1 h and then stimulated with LPS. Next, pro-inflammatory mediators and inflammation-related signal pathways were detected. As expected, our results showed that evodiamine notably ameliorated the inflammatory reaction and inhibit the activation of related signaling pathways of mMECs. All the results suggested that evodiamine inhibited inflammation by inhibiting the phosphorylation of AKT, NF-κBp65, ERK1/2, p38, and JNK thus the LPS-induced mastitis was ameliorated. These findings suggest that evodiamine maybe a potential drug for mastitis because of its anti-inflammatory effects.
Similar content being viewed by others
INTRODUCTION
In the dairy industry, cow mastitis is a significant disease with complex etiology and frequent occurrence, which affects the production and quality of dairy products [1]. After the occurrence of mastitis, the number of somatic cells and pH of milk are significantly changed, which reduces the yield and quality of milk. The economic loss caused by mastitis is the first among all kinds of diseases in dairy cows. There are many factors that cause inflammation in the animal body, such as atmospheric NH3 can cause jejunal fibrosis [2], hydrogen sulfide of air can aggravate inflammatory injury in trachea of chickens [3], and excess Li causes oxidative damage to promote the occurrence of inflammatory reactions in the carp kidney [4]. Mastitis is mainly caused by pathogenic microorganisms, especially gram-negative bacteria [5, 6]. After bacterial infection, a large amount of endotoxin can cause a strong immunogenic response in the mammary gland [7, 8]. The main component of gram-negative bacteria is lipopolysaccharide (LPS) [9], so LPS is considered an important factor in the establishment of animal model of inflammation [10,11,12]. Previous studies have shown that LPS bind to toll-like receptor 4 (TLR4) and activate multiple signal pathways such as nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) in in mammary epithelial cells [13]. This immune activity of mammary epithelial cells can promote the release of pro-inflammatory cytokines such as IL-1β and TNF-α, and the production of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (INOS), which obviously expand the inflammatory response and promote the aggravation of inflammation [14, 15].
In clinical application, the main treatment for mastitis is using of antibiotics, but this cannot effectively control the process of inflammation [16]. Recent studies have shown that the combined use of antibiotics and natural anti-inflammatory drugs can effectively alleviate the further development of mastitis [17, 18]. Compared with antibiotics, natural products often have strong anti-inflammatory functions meanwhile do not cause antibiotic residues and drug-resistant bacteria in milk, and no harmful residues into the food chain and affect human health [18, 19]. Consequently, the use of natural products in the treatment of mastitis is becoming increasingly widespread, and the search for new and effective natural anti-inflammatory drugs is the current hot spot in the treatment of dairy cow mastitis.
Evodiamine is an alkaloid component in the fruit of Evodia. Modern pharmacological studies have shown that a variety of biological activities of evodiamine play an important role in cardiovascular [20,21,22] and intestinal [23,24,25] diseases. In addition, evodiamine also has anti-tumor [26,27,28], hypoglycemic [29] and immunomodulatory [30] effects. However, how will it of evodiamine in mastitis has not been reported. Therefore, we constructed mastitis model in vivo and vitro by LPS to explore the protective effect and mechanism of evodiamine on mastitis.
MATERIALS AND METHODS
Animals
BALB/c mice are experimental animals used in this experiment; all experimental animals came from the Experimental Animal Center of Bethune Medical College, Jilin University (Jilin, China). All experimental animals and their operations were according to the guidelines that were formulated by the Jilin University Institutional Animal Care and Use Committee (approved on 27 February 2015, Protocol No. 2015047). During the animal study, the 8/9-week-old mice were randomly divided into the individually ventilated cages according to the combination of two females and a male, and were given sufficient food and water at 25 ± 1 °C. Until the females were pregnant, each male was removed.
Group Design and Construction of Mastitis Model
Evodiamine was taken from Shanghai Yuan Ye Bio-Technology Co. Ltd. (Shanghai, China) and have a purity of more than 98%. On the 5th and 7th days after childbirth, the mice were assigned to 5 groups stochastically: NT group (n = 6), evodiamine (50 mg/kg/day) group (n = 6), LPS group (n = 6) (dissolved in phosphate buffer (PBS)) (Sigma-Aldrich, St. Louis, Missouri, USA), LPS + evodiamine (50 mg/kg/day) group (n = 6), and LPS + dexamethasone (5 mg/kg/day) group (n = 6). The drug treatment group was given evodiamine (dissolved in normal saline). After separating experimental mice and young mice, they were fed with evodiamine or intraperitoneal injection of dexamethasone, and dexamethasone was used as a positive control [31, 32]. Evodiamine and LPS + evodiamine group were fed with evodiamine, and LPS + dexamethasone group was intraperitoneally injected with 0.1 mL dexamethasone. One hour later, the mice in LPS group, LPS + evodiamine group, and LPS + dexamethasone group were anesthetized and disinfected the surrounding skin of fourth pair of nipples with alcohol, and the nipples were removed at the 1 mm at the end of the milk duct to expose the milk duct. Ten micrograms of 0.2 mg/mL LPS was injected into each nipple catheter. After LPS injection 12 h, evodiamine was given to the mice in the evodiamine and LPS + evodiamine group. After another 12 h, the mice were sacrificed, and the mammary glands were collected.
Histopathological Examination of Mammary Glands
After the mammary glands of all experimental mice were separated and collected, an appropriate amount of mammary gland tissue was fixed, dehydrated, and transparent, and then embedded into paraffin blocks. Then, the paraffin blocks were fixed on the slicer, and 5 μm slices were cut, then dewaxed, hematoxylin–eosin stained, and observed under an optical microscope.
Tissue Homogenates and MPO Assay
A small piece of mammary gland was weighed and homogenized with hepes-free acid (HEPES) added in a ratio of 1:4. After centrifugation at 13,000 rpm for 30 min, the supernatant was collected for enzyme-linked immunosorbent assay (ELISA), and then the precipitate was homogenized with cetyltrimethylammonium chloride (CTAC). After centrifugation at 13,000 rpm for 30 min, the supernatant was diluted 10 times and reacted with a substrate containing TMB 3 mM, resorcin 6 mM, and 3% H2O2. The reaction was terminated with 2 M H2SO4, and finally, the OD value was measured at 450 mm.
ELISA
After getting the liquid supernatant collected for the first time in the MPO experiment, the levels of pro-inflammatory factors TNF- α and IL-1 β in mammary glands were detected as suggested by mouse ELISA kits (Biolegend, San Diego, CA, USA). First, the 96-well plate was coated with primary antibody at 4 °C overnight, washed with washing solution 4 times and then sealed with sample diluent at room temperature for 1 h, then washed the plate 4 times, added the sample to be tested, and shaken at room temperature. After 2 h, the plate was washed with washing solution, diluted avidin-horseradish peroxidase (HRP) solution was added, and TMB substrate color developing solution was added after shaking at room temperature for 30 min. After the color was developed, the reaction was terminated with 2 M H2SO4. Finally, the OD value was measured at 450 mm.
Cell Culture
The mouse mammary epithelial cells (mMECs) were acquired from the American Type Culture Collection (ATCC, ATCC® CRL-3063™, Rockville, MD, USA). They were cultured in different size cell culture plate (Life Science, Oneonta, NY, USA) The cell culture medium we used is Dulbecco’s modified Eagle medium (DMEM) (Gibco, Grand Island, NY 14,072, USA) (Clark Bioscience, Richmond, VA, USA) and it is containing 10% fetal bovine serum (FBS) (Clark Bioscience, Richmond, VA, USA). The mMECs are cultured in a humidified incubator at 37 °C containing 5% CO2, and the medium is changed every 2 days.
Cell Activity Assay
The cell viability assay of evodiamine in mMECs was detected by CCK-8 assay (Saint-Bio, Shanghai, China). The cells with 100 mL per well were divided into 8 groups to be added to the 96-well plate. The cells were treated with evodiamine in different concentrations (2.5, 5, 10, 20, 50, 100 mM) for 24 h. Then, 10 µL CCK-8 was added to each well, and the absorbance peak was detected at 450 mm.
Cell Experimental Design
When mMECs grow to 80% in the Petri dish, they are randomly divided into different groups: NT group, 10 mM evodiamine group, LPS group, and LPS + evodiamine (5 mM, 10 mM) treatment groups. When the cells grew to a density of about 80%, the serum-free medium was used instead of the serum-containing medium. Four hours later, different concentrations of evodiamine were respectively added to the Petri dish of 10 mM evodiamine group and LPS + evodiamine (5 mM, 10 mM) groups. One hour later, the cells were stimulated with LPS (1 μg/mL) equally in LPS group and LPS + evodiamine (5 mM, 10 mM) groups. Then, after 4 h, the cells were collected.
Real-Time PCR
The mRNA expression levels of IL-1β and TNF-α in mMECs were quantitatively detected by real-time PCR. After the cells were fully lysed with TRIzol (Invitrogen, Carlsbad, CA, USA), the total RNA of the cells was extracted [33]. Then, the reverse-transcribed (RT) sequence was synthesized by reverse transcription of 2 μg total RNA using the PrimeScript™ Kit (TaKaRa, Kyoto, Japan). RT-PCR analysis of gene expression was performed on the CFX96 system (Bio-Rad, Hercules, CA, USA) using cDNA and SYBR® Green Premix Ex Taq™ (TaKaRa, Kyoto, Japan) with the recommendation of manufacturer. The mRNA expression of TNF-α IL-1β, COX-2, and iNOS were normalized to with the mRNA expression of β-actin. The primer sequences are shown in Table 1.
Western Blot Analysis
Mammary tissues or mMECs have been lysed with a lysis pad. (Beyotime, Shanghai, China) by radio immunoprecipitation assay (RIPA). After centrifugation, the supernatant was collected to separate total protein, and BCA Protein Assay Kit (Beyotime, Shanghai, China) was used to determine the protein concentration. Through 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE),the protein was separated and then transferred to PVDF (Millipore, Darmstadt, Germany) membrane [34]. After sealed with 5% skim milk for 2 h, the PVDF membranes were incubated with a primary antibody (AKT (1:1000), phosphor-AKT (1:1000), ERK1/2 (1:1000), phospho-ERK1/2 (1:1000), JNK (1:1000), phosphor-JNK (1:1000), p38 (1:1000). phospho-p38 (1:1000), iNOS (1:1000), NF-κB p65 (1:1000), phospho-NF-κB p65 (1:1000), COX-2 (1:500), β-actin (1:5000) (Cell Signaling Technology, Beverly, MA, USA)) were incubated in 4 °C overnight [35]. Then, the PVDF membrane bound by antibody and protein was washed with Tris buffer saline Tween-20 (TBST) solution for 5 times, each time for 10 min, and then incubated with secondary antibody goat anti-rabbit antibody (1:6000) or goat anti-mouse antibody (1:6000) (Santa Cruz, California, USA) at 25 °C for 1 h, and then washed with TBST solution for 10 min for 5 times. Based on the manufacturer’s instructions, the specific protein strips were obtained using the Enhanced Chemiluminescence Detection Kit (Beyotime, Shanghai, China).
Statistical Analysis
A software called GraphPad Prism7 (Manufacturer, La Jolla, CA, USA) was used to process all experimental data. The experimental animals were randomly divided into 5 groups. In animal experiments, histological analysis was carried out by blinded manner. All data are represented by an average of means ± SEM, as shown in the figure legends. The differences between groups were compared by using one-way analysis of variance (# significant compared with NT group and * significant compared with LPS group, *p < 0.05, **p < 0.01, ***p < 0.001).
RESULTS
Evodiamine Improves LPS-Induced Mastitis in Mice
To explore the effect of evodiamine on mastitis mice, mouse mammary gland tissues from various treatment groups were collected for the following experiments. First of all, when the samples were collected, we found that evodiamine significantly reduced the redness and swelling of breast tissue caused by LPS (Fig. 1a). The HE staining results indicated that there were no abnormal histopathological changes in NT (no-treatment) group and evodiamine group, but there were congestion and swelling of mammary tissues acini and a large number of neutrophils infiltration in LPS group. Evodiamine and dexamethasone (DXMS) could dramatically alleviate the histopathological changes in mammary tissues induced by LPS (Fig. 1a, b). Myeloperoxidase (MPO) is a sign of inflammatory cell infiltration [36]. The detection of MPO activity in mammary tissues showed that the MPO activity in breast tissue increased significantly after LPS induction. However, evodiamine and DXMS significantly reduced MPO activity (Fig. 1c). Moreover, the above effects of evodiamine were more obvious than those of DXMS.
Evodiamine improves LPS-induced mastitis in mice. All mice were randomly divided into 5 groups: NT (no-treatment) group, evodiamine group, LPS group, LPS + evodiamine group, and LPS + dexamethasone (DXMS) group (n = 6). a The pictures and HE staining of the mammary tissues in different groups. b Histopathological score of mammary tissues. c Effect of evodiamine on MPO activity in mammary tissues. The values were presented as the means ± SEM of six independent experiments (n = 6). #p < 0.05 vs. NT group; ***p < 0.001 vs. LPS group
Evodiamine Reduces Inflammation in LPS-Induced Mouse Mastitis
Pro-inflammatory mediators, such as IL-1 β, TNF- α, COX-2, and iNOS play may major roles in the inflammation process [37]. The ELISA was used to detect the production of proinflammatory cytokines (IL-1 β and TNF-α) and the Western blot was used to detect the protein levels of proinflammatory enzymes (COX-2 and iNOS) in mammary gland. The results showed that the levels of IL-1β, TNF-α, COX-2, and iNOS protein in mammary tissues of LPS group were significantly higher than those in the NT group (Fig. 2a–e). Compared with the LPS group, the expression of pro-inflammatory mediators in LPS + evodiamine group decreased significantly. These results indicated that evodiamine can inhibit expression of pro-inflammatory mediators in LPS-induced mastitis mice.
Evodiamine reduces inflammation in LPS-Induced mouse mastitis. The secretions of IL-1β a and TNF-α b in the homogenate of mouse mammary glands were detected by ELISA. The protein levels of COX-2 c, d and iNOS c, e were measured by Western blot and the expression of densitometry quantitation with β-actin as an internal control. Data are presented as mean ± SEM (n = 3). #p < 0.05 vs. NT group; *p < 0.05, **p < 0.01, ***p < 0.001 vs. LPS group
Evodiamine Decreases NF-κB and MAPK Signaling Pathways Phosphorylation in LPS-Induced Mouse Mastitis
The expression of pro-inflammatory mediators is closely related to the activation of the NF-κB signaling pathway. In order to investigate the regulatory effect and anti-inflammatory mechanism of evodiamine in mammary tissues, the NF-κB and AKT activation (upstream kinase of NF-κB) were tested. The results of western blot showed that the LPS-induced phosphorylation of NF-κB p65 and AKT were obviously inhibited by evodiamine (Fig. 3a–c). The MAPK signaling pathway is also important in the occurrence and development of inflammation. Therefore, we detected the effects of evodiamine on ERK1/2, p38, and JNK phosphorylation. Similarly, the results showed that the evodiamine significantly inhibited LPS-induced phosphorylation of ERK1/2, p38 and JNK in the mammary tissues (Fig. 3a, d–f).
Evodiamine decreases NF-κB and MAPK signaling pathways phosphorylation in LPS-induced mouse mastitis. The protein levels of p-AKT a, b, p-NF-κB p65 a, c, p-ERK1/2 a, d, p-p38 a, e and p-JNK a, f in the mammary tissues were measured by Western blot. Data were presented as mean ± SEM (n = 3). #p < 0.05 vs. NT group; *p < 0.05, **p < 0.01, ***p < 0.001 vs. LPS group
Effect of Evodiamine on mMECs Viability
To test whether evodiamine has toxicity to mMECs, different concentrations of evodiamine were used to stimulate mMECs for 24 h. The cytotoxicity of evodiamine on mMECs was analyzed by CCK-8 assay. As shown in Fig. 4b, the evodiamine is not toxic to mMECs at concentrations below 20 μM. So, a concentration of 5, 10 µM were chosen for the next experiments.
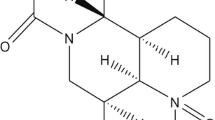
Effect of evodiamine on mMECs viability. a Chemical structure of evodiamine. b Effects of evodiamine on the cell viability of mMECs cultured with different concentrations of evodiamine (1.25, 2.5, 5, 10, and 20 µM). mMECs viability were determined by CCK-8 assay. Data are presented as mean ± SEM (n = 6). #p < 0.05 vs. NT group
Evodiamine Alleviates LPS-Induced Inflammatory Response in mMECs
Since mMECs is the sentinel cell in the mammary, it first recognizes pathogen-associated molecular patterns, such as LPS, in the early stage of mastitis. LPS-stimulated mMECs were used as an inflammatory cell model of mastitis. After mMECs was stimulated by LPS, the results showed that the mRNA levels of IL-1 β (Fig. 5b), TNF-α (Fig. 5c), COX-2 (Fig. 5d), and iNOS (Fig. 5e) increased significantly, and evodiamine (5, 10 μM) inhibited this response. Compared with NT group, iNOS (Fig. 6a, b) and COX-2 (Fig. 6a, c) protein level in LPS group significantly increased, and evodiamine (5, 10 μM) significantly inhibited this reaction.
Evodiamine alleviates expression of pro-inflammatory mediators in LPS-induced mMECs. The mRNA levels of IL-1β a, TNF-α b, COX-2 c, and iNOS d in the mMECs including NT group, evodiamine group, LPS group, and LPS + evodiamine (5, 10 µM) group were measured by real-time PCR. Data are presented as mean ± SEM (n = 3). #p < 0.05 vs. NT group; **p < 0.01, ***p < 0.001 vs. LPS group
Evodiamine alleviates protein levels of COX-2 and iNOS in LPS-induced mMECs. The protein levels of COX-2 (a, b) and iNOS (a, c) were measured by Western blot and the expression of densitometry quantitation with β-actin as an internal control. Data are presented as mean ± SEM (n = 3). #p < 0.05 vs. NT group; **p < 0.01 vs. LPS group.
Evodiamine Reduces NF-κB and MAPK Signaling Pathways Phosphorylation in LPS-Stimulated mMECs
In the model of LPS-induced mouse mastitis, we found that evodiamine could significantly inhibit the activation of AKT, NF-κB p65, ERK1/2, p38, and JNK in mammary tissue. We detect the effect of evodiamine (5, 10 μM) on the phosphorylation of AKT, NF-κB p65, and MAPK signaling pathways in LPS-stimulated mMECs by western blot to determine whether the effect of evodiamine on mastitis is linked to these signaling pathways; the results have shown that phosphorylation rates of AKT (Fig. 7a, b), NF-κB p65 (Fig. 7a, c), ERK1/2 (Fig. 7a, d), p38 (Fig. 7a, e), and JNK (Fig. 7a, f) were significantly increased in LPS group. As predicted, evodiamine (5, 10 μ M) could significantly inhibit this effect (Fig. 7).
Evodiamine on reduces AKT, NF-κB, and MAPK signaling pathways phosphorylation in LPS-stimulated mMECs. The phosphorylation of p-AKT a, b, p-NF-κB p65 a, c, p-ERK1/2 a, d, p-p38 a, e, and p-JNK a, f were measured by western blot. Data were presented as mean ± SEM (n = 3). #p < 0.05 vs. NT group; **p < 0.01 and ****p < 0.001 vs. LPS group
DISCUSSION
Evodiamine is the main alkaloid component of Evodia. Previous research has proven that evodiamine plays an important role in anti-inflammation and anti-infection [38]. This study in vivo revealed that evodiamine could inhibit the pathological changes in the mammary gland of LPS-induced mastitis by inhibiting the phosphorylation of AKT, NF-κB p65, and MAPK signal pathways, which further inhibiting the production of pro-inflammatory mediators. The results in vitro also showed that evodiamine could inhibited the inflammatory response and related signal pathways of mMECs.
Obvious pathological changes occurred in mammary gland tissue in LPS-induced mice mastitis. In this experiment, HE staining results showed that there had serious inflammatory reaction in LPS-induced mastitis included breast tissue wall obvious hyperplasia, breast acini hyperemia and edema, and lots of inflammatory cell infiltration, which is consistent with the phenomenon obtained by Gu et al. [39]. When treated with 50 mg/kg evodiamine, the number of inflammatory cells decreased and the state of the breast was basically normal. This shows that evodiamine can effectively reduce the inflammatory damage of breast tissue. After inflammation occurs, immune cells including neutrophils and monocytes [40, 41] recruit at the inflammatory site and release MPO, a defense enzyme with pro-oxidative which has pro-inflammatory properties [42, 43]. MPO is a feasible marker and an important therapeutic target for a variety of inflammatory diseases [44], including crescentic glomerulonephritis [45] and acute pneumonia [46]. In this study, the activity of MPO in evodiamine group was significantly lower than that in LPS group, which confirmed the decrease of inflammatory cell infiltration in mammary gland, indicating that evodiamine has a certain positive effect in LPS-induced mice mastitis. This may be associated to the inhibition of evodiamine on the excessive liberation of inflammatory factors induced by LPS.
Pro-inflammatory cytokines cause the release of other vasodilation-inducing chemicals and thus increasing the recruitment rate of inflammatory cells at the inflammatory site [47, 48]. It has been demonstrated that proinflammatory cytokines TNF-α and IL-1β play an important role in various types of inflammatory responses [49], including mastitis [50]. Among them, TNF-α can stimulate the expression of iNOS in immune cells to increase the secretion of nitric oxide (NO) in the body [51]. And TNF-α promotes the release of related prostaglandins by stimulating the expression of COX-2 in inflammatory cells [52]. Studies have shown that vasodilation during inflammationn is mainly mediated by nitric oxide (NO) and vasodilating prostaglandins [53]. Therefore, iNOS and COX-2 also play key role in LPS-induced mastitis model [31, 32]. In this study, we found that evodiamine can significantly reduce the release of TNF-α, IL-1 β, iNOS, and COX-2 in vivo and in vitro, and has a significant inhibitory effect on LPS-induced mastitis.
In inflammatory response, there are a lot of signal pathways involved. Some studies have shown that LPS binds to TLR4 and activates nuclear NF-κB and MAPKs through signal transduction, ultimately leading to increased transcription of pro-inflammatory cytokines such as TNF-α and IL-1β [54, 55]. Normally, NF-κB is located in the cytoplasm, until LPS induces activation of upstream kinase AKT resulting in phosphorylation of NF-κB p65, which can then be transferred to the nucleus and modulates transcription of pro-inflammatory mediators [56]. MAPK signaling pathways, including ERK, p38, and JNK subfamilies, regulate the expression of a variety of inflammatory factors and they have been identified as potential treatment targets of anti-inflammatory [57]. Previous studies have shown that evodiamine can alleviate severe pneumonia by inhibiting NF-κB and MAPK signaling pathways [58]. To determine if the anti-inflammatory effect of evodiamine in mastitis is linked to the NF-κB and MAPK signaling pathways, we detected the effects of evodiamine on AKT and NF-κB p65 and p38, ERK1/2, and JNK phosphorylation. Results in vivo and in vitro showed that LPS could significantly enhance the phosphorylation of AKT and NF-κB and the activation of MAPK signal pathways, which could be inhibited by evodiamine. These results suggest that the anti-inflammatory effect of evodiamine in mastitis is at least partially obtained by inhibition the phosphorylation of the signaling pathways AKT/NF-κB, ERK1/2, p38, and JNK.
To sum up, this study shows that evodiamine inhibits the production of pro-inflammatory mediator by down-regulating the phosphorylation of AKT/NF-κB p65 and MAPK signaling pathways in LPS-induced mastitis mice and mMECs. The results of this study provide a theoretical basis for the application of evodiamine in the treatment of mastitis, and provide a research direction for the role of evodiamine in other similar diseases.
Availability of Data and Materials
All data generated or analyzed during this study are included in this article.
References
Viguier, C., S. Arora, N. Gilmartin, K. Welbeck, and R. O’Kennedy. 2009. Mastitis detection: Current trends and future perspectives. Trends in Biotechnology 27: 486–493. https://doi.org/10.1016/j.tibtech.2009.05.004.
Jing, H., S. Wang, Y. Wang, N. Shen, and X.J. Gao. 2020. Environmental contaminant ammonia triggers epithelial-to-mesenchymal transition-mediated jejunal fibrosis with the disassembly of epithelial cell-cell contacts in chicken. Science of The Total Environment 726: 138686. https://doi.org/10.1016/j.scitotenv.2020.138686.
Song, N., et al. 2021. Hydrogen sulfide of air induces macrophage extracellular traps to aggravate inflammatory injury via the regulation of miR-15b-5p on MAPK and insulin signals in trachea of chickens. Science of The Total Environment 771 (145407): 2021. https://doi.org/10.1016/j.scitotenv.2021.145407.
Jing, H., Q. Zhang, and X. Gao. 2020. Excessive lithium of water induced a toxic effect on kidney via oxidative damage and inflammation in carp. Aquaculture 535:736282.
Ganda, E.K., R.S. Bisinotto, D.H. Decter, and R.C. Bicalho. 2016. Evaluation of an on-farm culture system (AccuMast) for fast identification of milk pathogens associated with clinical mastitis in dairy cows. PLoS ONE 11: e0155314. https://doi.org/10.1371/journal.pone.0155314.
Derakhshani, H., et al. 2018. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. Journal of Dairy Science 101: 10605–10625. https://doi.org/10.3168/jds.2018-14860.
Kayagaki, N., et al. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341: 1246–1249. https://doi.org/10.1126/science.1240248.
Pfalzgraff, A., L. Heinbockel, Q. Su, K. Brandenburg, and G. Weindl. 2017. Synthetic anti-endotoxin peptides inhibit cytoplasmic LPS-mediated responses. Biochemical Pharmacology 140: 64–72. https://doi.org/10.1016/j.bcp.2017.05.015.
Zhang, H.X., et al. 2016. H2S attenuates LPS-induced acute lung injury by reducing oxidative/nitrative stress and inflammation. Cellular Physiology and Biochemistry 40: 1603–1612. https://doi.org/10.1159/000453210.
Kauf, A.C., B.T. Vinyard, and D.D. Bannerman. 2007. Effect of intramammary infusion of bacterial lipopolysaccharide on experimentally induced Staphylococcus aureus intramammary infection. Research in Veterinary Science 82: 39–46. https://doi.org/10.1016/j.rvsc.2006.05.006.
Guo, W., et al. 2019. Licochalcone A protects the blood-milk barrier integrity and relieves the inflammatory response in LPS-induced mastitis. Frontiers in Immunology 10: 287. https://doi.org/10.3389/fimmu.2019.00287.
Ran, X., et al. 2020. Dioscin improves pyroptosis in LPS-induced mice mastitis by activating AMPK/Nrf2 and inhibiting the NF-kappaB signaling pathway. Oxidative Medicine and Cellular Longevity 2020: 8845521. https://doi.org/10.1155/2020/8845521.
Song, X., et al. 2014. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation 37: 1588–1598. https://doi.org/10.1007/s10753-014-9885-2.
Sordillo, L.M. 2018. Mammary gland immunobiology and resistance to mastitis. The Veterinary Clinics of North America Food Animal Practice 34: 507–523. https://doi.org/10.1016/j.cvfa.2018.07.005.
Serou, M.J., M.A. DeCoster, and N.G. Bazan. 1999. Interleukin-1 beta activates expression of cyclooxygenase-2 and inducible nitric oxide synthase in primary hippocampal neuronal culture: Platelet-activating factor as a preferential mediator of cyclooxygenase-2 expression. Journal of Neuroscience Research 58: 593–598.
Kromker, V., and S. Leimbach. 2017. Mastitis treatment-reduction in antibiotic usage in dairy cows. Reproduction in Domestic Animals 52 (Suppl 3): 21–29. https://doi.org/10.1111/rda.13032.
van Soest, F.J.S., E. Abbeloos, S. McDougall, and H. Hogeveen. 2018. Addition of meloxicam to the treatment of bovine clinical mastitis results in a net economic benefit to the dairy farmer. Journal of Dairy Science 101: 3387–3397. https://doi.org/10.3168/jds.2017-12869.
Pasca, C., et al. 2020. Efficacy of natural formulations in bovine mastitis pathology: Alternative solution to antibiotic treatment. Journal of Veterinary Research 64: 523–529. https://doi.org/10.2478/jvetres-2020-0067.
White, D.G., and P.F. Mcdermott. 2001. Emergence and transfer of antibacterial resistance. Journal of Dairy Science 84: E151–E155.
Zhao, T., et al. 2014. Pretreatment by evodiamine is neuroprotective in cerebral ischemia: Up-regulated pAkt, pGSK3β, down-regulated NF-κB expression, and ameliorated BBB permeability. Neurochemical Research 39: 1612–1620.
Yuan, S.M., et al. 2011. Evodiamine improves congnitive abilities in SAMP8 and APP(swe)/PS1(DeltaE9) transgenic mouse models of Alzheimer’s disease. Acta Pharmacologica Sinica 32: 295–302. https://doi.org/10.1038/aps.2010.230.
Meng, T., et al. 2020. Evodiamine inhibits lipopolysaccharide (LPS)-induced inflammation in BV-2 cells via regulating AKT/Nrf2-HO-1/NF-κB signaling axis. Cellular and molecular neurobiology 41: 115–127.
Zhao, Z., S. Gong, S. Wang, and C. Ma. 2015. Effect and mechanism of evodiamine against ethanol-induced gastric ulcer in mice by suppressing Rho/NF-кB pathway. International Immunopharmacology 28: 588–595.
Wen, B., C. Huang, L. Wu, and L.J.O. Hong. 2016. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. Therapy 9: 4121–4127.
Shen, P., et al. 2018. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-κB and NLRP3 inflammasome. Biomedicine & Pharmacotherapy 110: 786–795.
Wen, Z., S. Feng, L. Wei, Z. Wang, D. Hong, and Q. Wang. 2015. Evodiamine a novel inhibitor of the Wnt pathway inhibits the self-renewal of gastric cancer stem cells. International Journal of Molecular Medicine 36: 1657–1663.
Lin, L., L. Ren, L. Wen, Y. Wang, and J. Qi. 2016. Effect of evodiamine on the proliferation and apoptosis of A549 human lung cancer cells. Molecular Medicine Reports 14: 2832–2838.
Wei, L., X. Jin, Z. Cao, and W.J. Li. 2016. Evodiamine induces extrinsic and intrinsic apoptosis of ovarian cancer cells via the mitogen-activated protein kinase/phosphatidylinositol-3-kinase/protein kinase B signaling pathways. Journal of Traditional Chinese Medicine 36: 353–359.
Yu, L., Z. Wang, M. Huang, Y. Li, K. Zeng, J. Lei, and S. Zeng. 2016. Evodia alkaloids suppress gluconeogenesis and lipogenesis by activating the constitutive androstane receptor. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms 1859: 1100–1111.
Hu, H.Y., Z.Y. Song, L. Deng, and M.X. Zhang. 2008. Immunoregulatory effect of evodiamine in mice of various germlines. Journal of Experimental Hematology 16: 886–891.
Gong, Q., et al. 2018. Peiminine protects against lipopolysaccharide-induced mastitis by inhibiting the AKT/NF-kappaB, ERK1/2 and p38 signaling pathways. International Journal of Molecular Sciences 19: 2637. https://doi.org/10.3390/ijms19092637.
Li, Y., et al. 2018. Farrerol relieve lipopolysaccharide (LPS)-induced mastitis by inhibiting AKT/NF-kappaB p65 ERK1/2 and P38 signaling pathway. International Journal of Molecular Sciences 19: 1770. https://doi.org/10.3390/ijms19061770.
Ping, L., et al. 2019. CRTC2 is a key mediator of amino acid-induced milk fat synthesis in mammary epithelial cells. Journal of Agricultural and Food Chemistry 67: 10513–10520.
Li, X., P. Li, L. Wang, M. Zhang, and X. Gao. 2019. Lysine enhances the stimulation of fatty acids on milk fat synthesis via the GPRC6A-PI3K-FABP5 signaling in bovine mammary epithelial cells. Journal of Agricultural and Food Chemistry 67: 7005–7015.
Guo, W., et al. 2020. Butyrate alleviates oxidative stress by regulating NRF2 nuclear accumulation and H3K9/14 acetylation via GPR109A in bovine mammary epithelial cells and mammary glands. Free Radical Biology and Medicine 152: 728–742. https://doi.org/10.1016/j.freeradbiomed.2020.01.016.
Haegens, A., et al. 2009. Myeloperoxidase deficiency attenuates lipopolysaccharide-induced acute lung inflammation and subsequent cytokine and chemokine production. The Journal of Immunology 182: 7990–7996. https://doi.org/10.4049/jimmunol.0800377.
Kim, S.F., D.A. Huri, and S.H. Snyder. 2005. Inducible nitric oxide synthase binds S-nitrosylates and activates cyclooxygenase-2. Science 310: 1966–1970. https://doi.org/10.1126/science.1119407.
Liao, J.F., W.F. Chiou, Y.C. Shen, G.J. Wang, and C.F. Chen. 2011. Anti-inflammatory and anti-infectious effects of Evodia rutaecarpa (Wuzhuyu) and its major bioactive components. Chinese Medicine 6: 1–8.
Gu, B.B., J.F. Miao, Y.M. Zhu, Y.E. Deng, and S.X. Zou. 2009. Protective effect of retinoid against endotoxin-induced mastitis in rats. Inflammation Research 58 (2): 81–88.
Mantovani, A., M.A. Cassatella, C. Costantini, and S. Jaillon. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews Immunology 11: 519–531.
Shi, C., and E.G. Pamer. 2011. Monocyte recruitment during infection and inflammation. Nature Reviews Immunology 11: 762–774.
Bradley, P.P., R.D. Christensen, and G.J.B. Rothstein. 1982. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60: 618–622.
Klebanoff, S.J. 1970. Myeloperoxidase: Contribution to the microbicidal activity of intact leukocytes. Science 169: 1095–1097.
Aratani, Y. 2018. Myeloperoxidase: Its role for host defense inflammation and neutrophil function. Archives of Biochemistry and Biophysics 640: 47–52.
Kallenberg, C.J.A. 2014. Antineutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase. Autoantibodies 105–113.
Silvie, K., et al. 2016. Lung neutrophilia in myeloperoxidase deficient mice during the course of acute pulmonary inflammation. Oxidative Medicine and Cellular Longevity 2016: 5219056.
Medzhitov, R., and C.A. Janeway. 2002. Decoding the patterns of self and nonself by the innate immune system. Science 296: 298–300.
Ley, K., C. Laudanna, M.I. Cybulsky, and S. Nourshargh. 2007. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nature Reviews Immunology 7: 678–689.
Wojdasiewicz, P., Ł.A. Poniatowski, and D. Szukiewicz. 2014. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of Inflammation 2014: 561459.
Schmitz, S., M.W. Pfaffl, H.H.D. Meyer, and R.M. Bruckmaier. 2004. Short-term changes of mRNA expression of various inflammatory factors and milk proteins in mammary tissue during LPS-induced mastitis. Domestic Animal Endocrinology 26: 111–126.
Sanders, D.B., D.F. Larson, K. Hunter, M. Gorman, and B. Yang. 2001. Comparison of tumor necrosis factor-α effect on the expression of iNOS in macrophage and cardiac myocytes. Perfusion 16: 67–74.
Wallace, J.L. 2006. COX-2: A pivotal enzyme in mucosal protection and resolution of inflammation. The Scientific World Journal 6: 577–588.
Mark, K.S., W.J. Trickler, and D.W. Miller. 2001. Tumor necrosis factor-α induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. Journal of Pharmacology and Experimental Therapeutics 297: 1051–1058.
Ghosh, S., M.J. May, and E.B. Kopp. 1998. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annual Review of Immunology 16: 225–260.
Ren, Q., et al. 2019. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomedicine & Pharmacotherapy 122: 109772.
Manna, S.K. 2012. Double-edged sword effect of biochanin to inhibit nuclear factor kappaB: Suppression of serine/threonine and tyrosine kinases. Biochemical Pharmacology 83: 1383–1392.
Liu, E., T. Liang, X. Wang, S. Ban, L. Han, and Q. Li. 2015. Apoptosis induced by farrerol in human gastric cancer SGC-7901 cells through the mitochondrial-mediated pathway. European Journal of Cancer Prevention 24: 365–372.
Chen, X., S. Zhou, and H. Li. 2018. Evodiamine alleviates severe pneumonia induced by methicillin-susceptible Staphylococcus aureus following cytomegalovirus reactivation through suppressing NF-κB and MAPKs. International Journal of Molecular Medicine 42: 3247–3255.
Funding
Jilin Scientific and Technological Development Program, Grant/Award Number: Jilin Scientific and Technological Development Program, 20200201111JC, 20200703011ZP; National Natural Science Foundation of China, Grant/Award Number: 31873004; China Postdoctoral Science Foundation, Grant/Award Numbers: 2020M681043, 2020M681044; JLU Science and Technology Innovative Research Team.
Author information
Authors and Affiliations
Contributions
Yuanxi Yang, Xin Ran, and Shoupeng Fu designed experiments. Yuanxi Yang, Hefei Wang, and Yingsheng Chen carried out experiments. Yuanxi Yang, Zhanqing Yang, Wenjin Guo, and Guiqiu Hu analyzed experimental results. Yuanxi Yang, Xin Ran, Guiqiu Hu, and Wenjin Guo wrote the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors agree to submit the final version of manuscript for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuanxi Yang, Xin Ran, Hefei Wang, and Yingsheng Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, Y., Ran, X., Wang, H. et al. Evodiamine Relieve LPS-Induced Mastitis by Inhibiting AKT/NF-κB p65 and MAPK Signaling Pathways. Inflammation 45, 129–142 (2022). https://doi.org/10.1007/s10753-021-01533-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01533-9