Abstract
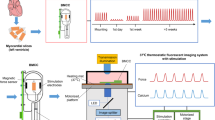
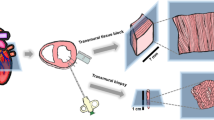
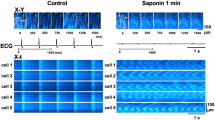
Ca2+ handling within cardiac myocytes underpins coordinated contractile function within the beating heart. This protocol enables high spatial and temporal Ca2+ imaging of ex vivo multicellular myocardial strips. The endocardial surface is retained, and strips of 150–300-µm thickness are dissected, loaded with Ca2+ indicators and mounted within 1.5 h. A list of the equipment and reagents used and the key methodological aspects allowing the use of this technique on strips from any chamber of the mammalian heart are described. We have successfully used this protocol on human, pig and rat biopsy samples. On use of this protocol with intact endocardial endothelium, we demonstrated that the myocytes develop asynchronous spontaneous Ca2+ events, which can be ablated by electrically evoked Ca2+ transients, and subsequently redevelop spontaneously after cessation of stimulation. This protocol thus offers a rapid and reliable method for studying the Ca2+ signaling underpinning cardiomyocyte contraction, in both healthy and diseased tissue.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
Bers, D. M. Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002).
Laver, D. R. Regulation of the RyR channel gating by Ca2+ and Mg2+. Biophys. Rev. 10, 1087–1095 (2018).
Wagner, E., Brandenburg, S., Kohl, T. & Lehnart, S. E. Analysis of tubular membrane networks in cardiac myocytes from atria and ventricles. J. Vis. Exp. 92, e51823 (2014).
Yue, L., Feng, J., Li, G. R. & Nattel, S. Transient outward and delayed rectifier currents in canine atrium: properties and role of isolation methods. Am. J. Physiol. Heart Circ. Physiol. 270, H2157–H2168 (1996).
Schneider-Warme, F., Johnston, C. M. & Kohl, P. Organotypic myocardial slices as model system to study heterocellular interactions. Cardiovasc. Res. 114, 3–6 (2018).
Watson, S. A. et al. Preparation of viable adult ventricular myocardial slices from large and small mammals. Nat. Protoc. 12, 2623–2639 (2017).
Perbellini, F. & Thum, T. Living myocardial slices: a novel multicellular model for cardiac translational research. Eur. Heart J. 41, 2405–2408 (2020).
Glukhov, A. V. et al. Direct evidence for microdomain-specific localization and remodeling of functional L-type calcium channels in rat and human atrial myocytes. Circulation 132, 2372–2384 (2015).
Brandenburg, S. et al. Axial tubule junctions control rapid calcium signaling in atria. J. Clin. Invest. 126, 3999–4015 (2016).
Hove-Madsen, L. et al. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation 110, 1358–1363 (2004).
Guatimosim, S., Guatimosim, C. & Song, L. S. Imaging calcium sparks in cardiac myocytes. Methods Mol. Biol. 689, 205–214 (2011).
Apati, A. et al. Calcium signaling in pluripotent stem cells. Mol. Cell. Endocrinol. 353, 57–67 (2012).
Lamont, C., Luther, P. W., Balke, C. W. & Wier, W. G. Intercellular Ca2+ waves in rat heart muscle. J. Physiol. 512, 669–676 (1998).
Wakayama, Y., Miura, M., Stuyvers, B. D., Boyden, P. A. & ter Keurs, H. E. Spatial nonuniformity of excitation-contraction coupling causes arrhythmogenic Ca2+ waves in rat cardiac muscle. Circ. Res. 96, 1266–1273 (2005).
Wier, W. G., ter Keurs, H. E., Marban, E., Gao, W. D. & Balke, C. W. Ca2+ ‘sparks’ and waves in intact ventricular muscle resolved by confocal imaging. Circ. Res. 81, 462–469 (1997).
Slabaugh, J. L. et al. Synchronization of intracellular Ca2+ release in multicellular cardiac preparations. Front. Physiol. 9, 968 (2018).
Lou, Q. et al. Transmural heterogeneity and remodeling of ventricular excitation-contraction coupling in human heart failure. Circulation 123, 1881–1890 (2011).
Kaneko, T., Tanaka, H., Oyamada, M., Kawata, S. & Takamatsu, T. Three distinct types of Ca2+ waves in Langendorff-perfused rat heart revealed by real-time confocal microscopy. Circ. Res. 86, 1093–1099 (2000).
Jones, J. S., Small, D. M. & Nishimura, N. In vivo calcium imaging of cardiomyocytes in the beating mouse heart with multiphoton microscopy. Front. Physiol. 9, 969 (2018).
Fujiwara, K., Tanaka, H., Mani, H., Nakagami, T. & Takamatsu, T. Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+–Ca2+ exchanger: simultaneous confocal recording of membrane potential and intracellular Ca2+ in the heart. Circ. Res. 103, 509–518 (2008).
Wasserstrom, J. A. et al. Variability in timing of spontaneous calcium release in the intact rat heart is determined by the time course of sarcoplasmic reticulum calcium load. Circ. Res. 107, 1117–1126 (2010).
Pillekamp, F. et al. Establishment and characterization of a mouse embryonic heart slice preparation. Cell. Physiol. Biochem. 16, 127–132 (2005).
Meyer, T., Stuerz, K., Guenther, E., Edamura, M. & Kraushaar, U. Cardiac slices as a predictive tool for arrhythmogenic potential of drugs and chemicals. Expert Opin. Drug Metab. Toxicol. 6, 1461–1475 (2010).
Bussek, A. et al. Cardiac tissue slices with prolonged survival for in vitro drug safety screening. J. Pharmacol. Toxicol. Methods 66, 145–151 (2012).
Brandenburger, M. et al. Organotypic slice culture from human adult ventricular myocardium. Cardiovasc. Res. 93, 50–59 (2012).
Kang, C. et al. Human organotypic cultured cardiac slices: new platform for high throughput preclinical human trials. Sci. Rep. 6, 28798 (2016).
Perbellini, F. et al. Investigation of cardiac fibroblasts using myocardial slices. Cardiovasc. Res. 114, 77–89 (2018).
Wen, Q. et al. Transverse cardiac slicing and optical imaging for analysis of transmural gradients in membrane potential and Ca2+ transients in murine heart. J. Physiol. 596, 3951–3965 (2018).
Wang, K. et al. Cardiac tissue slices: preparation, handling, and successful optical mapping. Am. J. Physiol. Heart Circ. Physiol. 308, H1112–H1125 (2015).
Camelliti, P. et al. Adult human heart slices are a multicellular system suitable for electrophysiological and pharmacological studies. J. Mol. Cell. Cardiol. 51, 390–398 (2011).
Bussek, A. et al. Tissue slices from adult mammalian hearts as a model for pharmacological drug testing. Cell. Physiol. Biochem. 24, 527–536 (2009).
Burdyga, T., Shmygol, A., Eisner, D. A. & Wray, S. A new technique for simultaneous and in situ measurements of Ca2+ signals in arteriolar smooth muscle and endothelial cells. Cell Calcium 34, 27–33 (2003).
Borisova, L., Wray, S., Eisner, D. A. & Burdyga, T. How structure, Ca signals, and cellular communications underlie function in precapillary arterioles. Circ. Res. 105, 803–810 (2009).
Borysova, L., Wray, S., Eisner, D. A. & Burdyga, T. How calcium signals in myocytes and pericytes are integrated across in situ microvascular networks and control microvascular tone. Cell Calcium 54, 163–174 (2013).
Burdyga, T., Borisova, L., Burdyga, A. T. & Wray, S. Temporal and spatial variations in spontaneous Ca events and mechanical activity in pregnant rat myometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 144, S25–S32 (2009).
Mumtaz, S., Burdyga, G., Borisova, L., Wray, S. & Burdyga, T. The mechanism of agonist induced Ca2+ signalling in intact endothelial cells studied confocally in in situ arteries. Cell Calcium 49, 66–77 (2011).
Borysova, L., Dora, K. A., Garland, C. J. & Burdyga, T. Smooth muscle gap-junctions allow propagation of intercellular Ca2+ waves and vasoconstriction due to Ca2+ based action potentials in rat mesenteric resistance arteries. Cell Calcium 75, 21–29 (2018).
Kagemoto, T. et al. Sarcomeric auto-oscillations in single myofibrils from the heart of patients with dilated cardiomyopathy. Circ. Heart Fail. 11, e004333 (2018).
Bagher, P. et al. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc. Natl Acad. Sci. USA. 109, 18174–18179 (2012).
Garland, C. J. et al. Voltage-dependent Ca2+ entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci. Signal. 10, 1–14 (2017).
Qiao, Y. et al. Multiparametric slice culture platform for the investigation of human cardiac tissue physiology. Prog. Biophys. Mol. Biol. 144, 139–150 (2019).
Fischer, C. et al. Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat. Commun. 10, 117 (2019).
Garger, J. C. et al. Guide for the Care and Use of Laboratory Animals 8th edn (National Academies Press, 2011).
Gadeberg, H. C. et al. Heterogeneity of t-tubules in pig hearts. PLoS One 11, e0156862 (2016).
McGrath, J. C. & Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br. J. Pharmacol. 172, 3189–3193 (2015).
Sasaki, D., Fujita, H., Fukuda, N., Kurihara, S. & Ishiwata, S. Auto-oscillations of skinned myocardium correlating with heartbeat. J. Muscle Res. Cell Motil. 26, 93–101 (2005).
Zhao, G., Joca, H. C., Nelson, M. T. & Lederer, W. J. ATP- and voltage-dependent electro-metabolic signaling regulates blood flow in heart. Proc. Natl Acad. Sci. USA. 117, 7461–7470 (2020).
Acknowledgements
We thank the British Heart Foundation (BHF) and Medical Research Council (MRC) for grant awards to R.A. enhancing this work (grant numbers BHF PG/18/49/33833, BHF IG/14/2/30991, BHF/PG/16/104/32652 and MRC MR/L012723/1). Collection of human biopsy specimens in Bristol was supported by the NIHR Bristol Biomedical Research Centre. In addition, this work was supported by British Heart Foundation grants to K.A.D. (grant numbers FS/08/033/25111, FS/13/16/30199, IG/13/5/30431 and PG/18/11/33552) and by the Oxford BHF Centre of Research Excellence (RE/13/1/30181). We thank Theodore Burdyga at the University of Liverpool for use of the custom-built chambers and general protocols for using them and Carsten Thorndahl and Rene Hemmel at DMT for the development and supply of the Confocal Cardiac Myograph. We thank the research nurses, laboratory technicians and surgeons at the Bristol Heart Institute, University Hospital Bristol-Weston NHS Foundation Trust and the Bristol Trials Centre (Clinical Trials and Evaluation Unit) at the University of Bristol. We also thank the staff and researchers at the University of Bristol Translational Biomedical Research Centre, a national research facility for large animals co-funded by the BHF and MRC. Finally, we express our full gratitude to all the patients taking part in this study.
Author information
Authors and Affiliations
Contributions
K.A.D. wrote the manuscript, collected and analyzed data and contributed to the optimization of the protocol. L.B. and Y.Y.H.N. contributed to writing the manuscript. L.B., Y.Y.H.N., E.S.W. and L.E.W. collected and analyzed data and contributed to the optimization of the protocol. E.F. collected data. R.A. provided all human and porcine specimens and contributed to the manuscript. All authors proofread the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Filippo Perbellini, Thomas Seidel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Burdyga, T. et al. Cell Calcium 34, 27–33 (2003): https://doi.org/10.1016/s0143-4160(03)00019-8
Borisova, L. et al. Circ. Res. 105, 803–810 (2009): https://doi.org/10.1161/CIRCRESAHA.109.202960
Borysova, L. et al. Cell Calcium 75, 21–29 (2018): https://doi.org/10.1016/j.ceca.2018.08.001
Supplementary information
Supplementary Video 1
Cutting and pinning of porcine RAA
Supplementary Video 2
Cutting of porcine RAA into myocardial strips
Supplementary Video 3
Isolation of rat RAA from a whole heart
Supplementary Video 4
Cutting and pinning of rat RAA
Supplementary Video 5
Cutting of rat RAA into myocardial strips
Supplementary Video 6
Mounting of a rat RAA myocardial strip into three types of imaging chambers (#1, #2 and #3)
Supplementary Video 7
Spontaneous Ca2+ waves in a human RAA myocardial strip
Supplementary Video 8
Spontaneous Ca2+ sparks in a human RAA myocardial strip
Supplementary Video 9
Spontaneous Ca2+ waves in a porcine RAA myocardial strip
Supplementary Video 10
Spontaneous Ca2+ waves in a rat RAA myocardial strip
Supplementary Video 11
Electrically evoked CaTs in a rat RAA myocardial strip
Supplementary Video 12
Simultaneous imaging of cardiac and endocardial endothelial cell Ca2+ in a rat RAA myocardial strip
Supplementary Video 13
Isolation, cutting, pinning and dissection of rat ventricles followed by cutting of myocardial strips from a rat left ventricle
Supplementary Video 14
Spontaneous Ca2+ waves in a rat left ventricle myocardial strip
Supplementary Video 15
Electrically evoked CaTs in a rat left ventricle myocardial strip
Source data
Source Data Fig. 2
Raw data.
Source Data Fig. 3
Raw data.
Source Data Fig. 4
Raw data.
Source Data Fig. 5
Raw data.
Source Data Fig. 6
Raw data.
Source Data Fig. 7
Raw data.
Source Data Fig. 9
Raw data.
Rights and permissions
About this article
Cite this article
Borysova, L., Ng, Y.Y.H., Wragg, E.S. et al. High spatial and temporal resolution Ca2+ imaging of myocardial strips from human, pig and rat. Nat Protoc 16, 4650–4675 (2021). https://doi.org/10.1038/s41596-021-00590-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00590-6
This article is cited by
-
A four-dimensional model for the information storage/output model of life
Nano Research (2023)
-
Quantum essence of particle superfluidity
Nano Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.