Abstract
Background
Docosahexaenoic acid (DHA) and arachidonic acid (AA) are important for fetal brain growth and development. Our aim was to evaluate the association between serum DHA and AA levels and brain volumes in extremely preterm infants.
Methods
Infants born at <28 weeks gestational age in 2013–2015, a cohort derived from a randomized controlled trial comparing two types of parenteral lipid emulsions, were included (n = 90). Serum DHA and AA levels were measured at postnatal days 1, 7, 14, and 28, and the area under the curve was calculated. Magnetic resonance (MR) imaging was performed at term-equivalent age (n = 66), and volumes of six brain regions were automatically generated.
Results
After MR image quality assessment and area under the curve calculation, 48 infants were included (gestational age mean [SD] 25.5 [1.4] weeks). DHA levels were positively associated with total brain (B = 7.966, p = 0.012), cortical gray matter (B = 3.653, p = 0.036), deep gray matter (B = 0.439, p = 0.014), cerebellar (B = 0.932, p = 0.003), and white matter volume (B = 3.373, p = 0.022). AA levels showed no association with brain volumes.
Conclusions
Serum DHA levels during the first 28 postnatal days were positively associated with volumes of several brain structures in extremely preterm infants at term-equivalent age.
Impact
-
Higher serum levels of DHA in the first 28 postnatal days are positively associated with brain volumes at term-equivalent age in extremely preterm born infants.
-
Especially the most immature infants suffer from low DHA levels in the first 28 postnatal days, with little increase over time.
-
Future research is needed to explore whether postnatal fatty acid supplementation can improve brain development and may serve as a nutritional preventive and therapeutic treatment option in extremely preterm infants.
Similar content being viewed by others
Introduction
Long-chain polyunsaturated fatty acids (LCPUFAs) play an important role in fetal brain development.1 Among PUFAs in the brain, the omega-3 fatty acid docosahexaenoic acid (DHA) accounts for the largest fraction (~40%).2 During the major growth spurt of the brain (from the second trimester of pregnancy until 18 months of age), the amount of DHA in the brain increases 30-fold.3 DHA is an important component of cell membranes, but its metabolites are also involved in neurogenesis, antiapoptotic effects, synaptic plasticity, and other phenomena.2 The omega-6 LCPUFA arachidonic acid (AA) is known for its structural function in cell membranes, signaling, specific neuroprotective protein activation, and formation of eicosanoids.4 DHA and AA accumulate in the fetal brain mainly during the third trimester,1 a critical period for fetal brain development. Extremely preterm infants, already at risk for an adverse neurodevelopmental outcome, are no longer supplied by the placenta, but rely on parenteral and enteral nutrition for their DHA and AA intake. Current parenteral and enteral nutrition may not be sufficient to supply an adequate amount of DHA and AA to extremely preterm infants,5,6 thus putting them at risk for decreased fatty acid levels.5,7,8 This is demonstrated as early as the first week after birth,7 and by DHA and AA levels available for accretion at 6 weeks of age that were ~60% and 85% lower than provided in utero, respectively.5 The most immature infants are especially at risk, as DHA and AA levels seem to depend on gestational age (GA).9 Lapillonne et al. found that preterm infants have lower erythrocyte DHA levels than term infants, and erythrocyte DHA levels depend on the type of enteral intake.8,10,11 Around term-equivalent age (TEA), preterm infants who received standard formula, LCPUFA-enriched formula, or breastfeeding had 52%, 14%, and 31% lower erythrocyte DHA levels, respectively, than term infants.8
Low serum AA and DHA levels are associated with multiple morbidities, such as late-onset sepsis and chronic lung disease, respectively,7 which may be explained by the associations between DHA and AA levels and inflammation. Lower DHA and AA cord blood levels are associated with increased levels of the pro-inflammatory cytokine interleukin-6, and lower postnatal DHA levels are associated with increased early systemic inflammation in extremely preterm infants.12 Systemic inflammation plays a major role in the development of white matter injury in preterm infants.13 In one study, higher single erythrocyte DHA levels around birth and TEA in preterm infants were associated with greater brain volumes.14 However, little is known about the effect of serum fatty acids levels during the first postnatal month on brain volumes at TEA. Brain volumes at TEA are correlated with neurodevelopmental outcome,14,15,16 which indicates their potential as biomarkers for future development.
In a randomized controlled clinical trial, parenteral nutrition with fish oil, containing DHA, had no effect on morbidity, but it suppressed serum AA levels.17 In addition, lower serum AA levels were associated with severe retinopathy of prematurity.18 For this part of the study, all infants are analyzed as one group, regardless of study treatment. The aim of this study is to evaluate whether serum levels of DHA and AA are associated with brain volumes at TEA in extremely preterm infants and to evaluate whether fatty acid serum levels are influenced by GA.
Methods
Study design
This cohort of infants was derived from a randomized, controlled, open-label trial, evaluating the effect of two parenteral emulsions on retinopathy of prematurity, short-term morbidity, and growth. The trial was registered at ClinicalTrials.gov (NCT02760472). Infants were randomized into receiving either SMOFlipid® (Fresenius Kabi, Bad Homburg, Germany), containing 15% fish oil with omega-3 LCPUFAs, or Clinoleic® (Baxter, Deerfield, Illinois), an olive-oil based emulsion. The study was approved by the Regional Ethical Board of Gothenburg. Informed consent from parents or guardians was obtained in writing before the inclusion of the infant in the trial. The primary study outcome was previously described by Najm et al., showing no differences in growth (weight, length, and head circumference) or morbidity between the two groups.17 Brain development in relation to serum fatty acid levels was a secondary outcome measure in this trial.
Patients
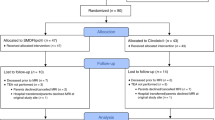
Infants born at <28 weeks GA from April 2013 until September 2015 at Sahlgrenska University Hospital in Gothenburg, Sweden, were eligible for inclusion in the original clinical trial. For this study, additional inclusion criteria were availability of magnetic resonance (MR) images at TEA of sufficient quality for segmentation, and blood samples at postnatal days 1 and 28. MR acquisition and blood sampling are described in detail below. Exclusion criteria were major congenital malformations. Of the 138 eligible patients, 90 were included in the study. In 24 patients, no MR images were acquired (13 patients died prior to scanning and 11 patients did not undergo scanning due to parental refusal). In the 66 remaining infants, 52 infants had MR images of sufficient quality for segmentation. Reasons for exclusion of MR examinations were severe motion artifact (n = 5), missing T2-weighted (T2w) images (n = 3), field of view not covering the complete brain parenchyma (n = 4), and a combination of two or three of the previously mentioned reasons (n = 2). Due to missing blood sampling for fatty acid levels (n = 4), 48 infants were included in the final analysis (Fig. 1). There were no statistically significant differences in baseline characteristics between included and excluded surviving infants (data not shown).
Of the 48 included patients, 22 received SMOFlipid® and 26 received Clinoleic®. There were no statistically significant differences in baseline characteristics, nor in brain volumes between the treatment groups (data not shown). DHA area under the curve (AUC) was significantly higher in the SMOFlipid® group than in the Clinoleic® group (mean [SD] 11.0 [1.4] vs 9.9 [1.6], p = 0.019). Since the aim of our study was to evaluate DHA serum levels (combined to form an AUC) and not the effect of DHA-enriched parenteral nutrition, all infants were analyzed as one group and no further distinction between the type of parenteral nutrition was made in the analyses.
Nutritional management
Nutritional management has been described previously.17,19 In short, all patients received parenteral and enteral nutrition according to routine clinical care. Parenteral nutrition was started as soon as possible after birth, with a final lipid dosing strategy of 2–3 g/kg per day. Minimal enteral feeding was started within 3 h after birth, with a gradual increase in volume. Enteral nutrition consisted of own mother’s milk or donor milk, with individual fortification.
Blood sampling
Blood samples drawn at postnatal days 1, 7, 14, and 28 were used in the analyses. Serum phospholipid fatty acid levels (expressed as mol%) were previously measured.17 For this study no new chemical analysis was performed. In serum/plasma, LCPUFAs are found in lipoprotein particles, particularly enriched in the phospholipid fraction. In preterm infants, the plasma proportion of the main phospholipid phosphatidylcholine (PC) containing DHA and AA is linearly related to erythrocyte PC-DHA and PC-AA, respectively, and likely also reflect LCPUFAs status in peripheral organs including the brain.20 An AUC for the first 28 days was calculated using the trapezoidal method in patients who had at least measurements at both postnatal days 1 and 28. AUC represents a time-adjusted sum of all four individual measurements.
Morbidity
Short-term morbidity, including necrotizing enterocolitis, persistent ductus arteriosus, sepsis, and bronchopulmonary dysplasia, were defined as previously described17 and were registered from the patient’s clinical record. Intraventricular hemorrhage was classified by serial cranial ultrasound imaging according to Papile et al.21 Significant brain injury was defined as intraventricular hemorrhage (grades III and IV), white matter lesions (focal signal abnormality score ≥2 according to Kidokoro et al.22), cerebellar hemorrhage (signal abnormality score ≥2 according to Kidokoro et al.22), and/or cystic lesions (cystic lesion score ≥3 according to Kidokoro et al.22).
A detailed description of randomization, nutritional strategy, parenteral and enteral lipid administration, and blood sampling and analysis was previously published.17,18
MR image acquisition
Around TEA (median postmenstrual age in weeks [interquartile range]: 43.0 [2.93]), all included infants were examined on a 3-Tesla system (750 W, GE Medical Systems, Waukesha, WI) using a 19- or 32-channel head coil. Infants were sedated using oral chloral hydrate (35 mg/kg). For hearing protection, a combination of purpose-made in-ear and over-ear sound absorption devices was used. All infants were closely monitored by a trained nurse or physician, including respiratory rate and oxygen saturation, as well as heart rate throughout the whole procedure.
The routine acquisition protocol included axial 2D T2w, T1 FLAIR, 3D T2w, 3D T1-weighted, diffusion-weighted, and 3D susceptibility-weighted images. For the volumetric analysis, only T2w images were used. The 2D T2w acquisition parameters were: slice thickness 3 mm; repetition time 9278 ms; echo time 74.5 ms. The 3D fast spin-echo T2w acquisition parameters were: slice thickness 0.8 mm; repetition time 2740–3000 ms; echo time 81–125 ms.
MR image post processing
All T2w images were processed, including multiple acquisitions from a single infant during the same MR imaging session (in total n = 141 acquisitions). The images from each single acquisition (whether 2D or direct 3D) were combined into one 3D image volume, referred to in the following as image stack. Regional brain volumes were measured on the basis of the anatomical segmentation procedure described by Makropoulos et al.23 In summary, multiple brain regions were identified based on a manually segmented atlas,24 with automatic atlas-based anatomical segmentation using the DrawEM (Developing brain Region Annotation With Expectation-Maximization) software module of the Medical Image Registration Toolkit.23,25 An experienced imaging scientist (R.A.H.) assessed the segmentations, using a custom-designed quality scoring protocol, and excluded image stacks when quality deficiencies implied that volume calculations would be unreliable. If more than one image stack from the same infant was segmented with sufficient quality, only the segmentation with the highest quality was included in further analysis. This resulted in 52 segmentations from 52 infants. Merged volumes of six brain regions (total brain [i.e., total intracranial volume without cerebrospinal fluid], white matter, cortical gray matter, deep gray matter, cerebellum, and ventricles [i.e., lateral ventricles]) were generated by summation of selected individual regions.
Statistical analysis
Data were analyzed using IBM SPSS Statistics 25 (IBM Corp., Armonk, NY). Linear regression was used to evaluate the association between serum fatty acids (DHA AUC and AA AUC) and the six merged brain region volumes. In all models, results were corrected for postmenstrual age at the time of MR scanning (weeks). The following confounders were included in the initial analysis: significant brain injury (yes/no), weight standard deviation score (SDS) at time of MR scanning, GA at birth (weeks), and change in weight SDS from birth until the time of MRI. Confounders were excluded from the final multivariable regression model if they were not significant. Weight SDS scores were calculated according to Niklasson et al.26 DHA for GA curves were created to evaluate the effect of low DHA levels on brain volumes. All available DHA AUC of the infants from the original cohort (n = 73) were used to determine the 50th percentile of DHA AUC for each GA. Infants with DHA AUC below the 50th percentile for GA were classified as “lower DHA AUC,” and this variable was included in the linear regression model. All models met the assumptions for linear regression analysis (homoscedasticity, normally distributed residuals, independence of measurements, and linearity). Independent-samples t test and χ2 tests were used to compare baseline characteristics between included and excluded surviving infants and between infants who received SMOFlipid® versus Clinoleic®. To evaluate whether DHA and AA levels were influenced by GA, two GA groups were created. The threshold was set at 25 weeks GA, based on clinical morbidity and mortality data.27,28 Levels of DHA from day 7 onwards for infants with GA < 25 weeks and infants with GA ≥ 25 weeks were analyzed using mixed models in R (https://www.r-project.org/). For all analyses, a p value < 0.05 was considered statistically significant.
Results
Patient characteristics
The clinical characteristics of all 48 included infants are shown in Table 1. Nutritional characteristics are shown in Table 2.
Brain volumes and fatty acid AUC
Mean brain volumes (cm3) are shown in Table 3. In the final regression models for all brain volumes, change in weight SDS from birth until the time of MR scanning was excluded, since it was not significant. GA at birth was significantly associated with serum DHA AUC (Fig. 2). Inclusion of GA in the model together with DHA made the model unstable, and GA was therefore not included in the final model. Thus, the final multivariable regression model for total brain volume, cortical gray matter volume, and cerebellar volume consisted of postmenstrual age at the time of MR scanning, significant brain injury, and weight SDS at the time of MR scanning, and was completed with DHA AUC or AA AUC (Table 4). In the multivariable regression models for deep gray matter volume, white matter volume, and ventricular volume, one or both of the remaining confounders (significant brain injury and weight SDS at the time of MR scanning) were not significant and were therefore excluded from the final model (Table 4).
DHA AUC was positively associated with total brain volume (B = 7.966, 95% confidence interval (CI) 1.837–14.095, p = 0.012, Fig. 3a), cortical gray matter volume (B = 3.653, 95% CI 0.251–7.055, p = 0.036, Fig. 3b), deep gray matter volume (B = 0.439, 95% CI 0.091–0.787, p = 0.014, Fig. 3c), cerebellar volume (B = 0.932, 95% CI 0.333–1.530, p = 0.003, Fig. 3d), and white matter volume (B = 3.373, 95% CI 0.504–6.241, p = 0.022, Fig. 3e) (Table 4). No association with ventricular volume was found (B = −0.341, 95% CI −1.231 to 0.549, p = 0.444). In addition, no associations between AA AUC and any of the brain volumes were found (Table 4).
Overview of docosahexaenoic acid area under the curve (AUC) associations with total brain volume (a), cortical gray matter volume, GMc (b), deep gray matter volume, GMd (c), cerebellar volume, Crb (d), and white matter volume, WM (e). B and p values represent the complete multivariable regression model, which corrects for one or more of the following: postmenstrual age at the time of magnetic resonance imaging, significant brain injury, and weight standard deviation score at the time of magnetic resonance imaging. GA gestational age, Vnt ventricles.
Since GA was excluded from the final DHA AUC model, additional analyses with “lower DHA AUC” in the regression model together with GA (and the previously described variables) were performed. Twenty-one infants were classified as “lower DHA AUC.” Linear regression analysis showed negative associations between “lower DHA AUC” and total brain, cortical gray matter, deep gray matter, white matter, and cerebellar volume, although not significant. The association between “lower DHA AUC” and cerebellar volume nearly reached significance (p = 0.054). Additional analyses were performed with GA in the model while omitting DHA from the model, to obtain more information on the interplay between GA, DHA, and brain volumes. The models with GA as an independent variable were highly comparable to the models with DHA as an independent variable (Appendix 1).
DHA and AA levels over time
For these results, the individual fatty acids measurements at postnatal days 1, 7, 14, and 28 were used, instead of the AUC. During the first postnatal week, DHA levels declined drastically, after which they slowly increased during the second, third, and fourth postnatal week. However, after 28 postnatal days, DHA levels were still below the baseline levels of postnatal day 1 (Fig. 4a). Similarly, AA levels dropped in the first postnatal week, but did not increase during the first 28 postnatal days (Fig. 4b). Except for the first postnatal day, GA had a significant effect on DHA levels, but not on AA levels (Figs. 2 and 4). Mixed model analysis showed that DHA levels increased significantly (0.026 mol% per day, p < 0.001) between days 7 and 28, and that DHA levels were significantly lower in infants with GA < 25 weeks (n = 15) (0.48 mol%, p < 0.001) compared to infants with GA ≥ 25 weeks (n = 33). Mixed-model analysis of AA did not show a statistically significant increase over time, nor a difference between GA groups.
Docosahexaenoic acid (a) and arachidonic acid (b) levels at postnatal (PN) days 1, 7, 14, and 28 for infants with gestational age (GA) < 25 weeks (n = 15) and ≥25 weeks (n = 33). The difference in docosahexaenoic acid levels between the GA groups was 0.48 mol%, p < 0.001. Docosahexaenoic acid levels increased from PN day 7 onwards (0.026 mol% per day between PN days 7 and 28, p < 0.001). Arachidonic acid levels did not differ significantly between GA groups and no effect of PN age between 7 and 28 days could be demonstrated.
Discussion
To our knowledge, this is the first study to evaluate the association between multiple serum DHA and AA measurements in the first 28 postnatal days and brain volumes at TEA in extremely preterm infants. This study showed that higher serum levels of DHA, but not AA, during the first 28 postnatal days were associated with the increased total brain, cortical gray matter, deep gray matter, cerebellar, and white matter volumes at TEA in infants born extremely preterm. In addition, infants with GA < 25 weeks had significantly lower serum levels of DHA between postnatal days 7 and 28 than infants with GA ≥ 25 weeks.
Postnatal DHA and AA development
The pattern of postnatal DHA and AA development in our cohort is in line with previous findings. Two studies described a postnatal decrease in DHA and AA levels during the first week in preterm infants (GA < 30 weeks and GA 24–34 weeks).7,29 This initial decrease in LCPUFAs indicates that the amount of LCPUFAs extremely preterm infants receive may be insufficient, which is a major concern considering their high requirement.
Our results show an interesting GA-dependent difference in DHA levels. Infants with GA < 25 weeks have a more severe drop in serum DHA during the first week of life than infants with GA ≥ 25 weeks, and this difference persists throughout the first 28 postnatal days. Most fetal DHA accumulation takes place during the third trimester of pregnancy, with more DHA being incorporated in the brain for every week of gestation.1 Consequently, at corresponding chronological age, more immature infants have accumulated less DHA in the brain, as well as in other tissues including liver and retina, than more mature infants. We speculate that in order to meet the high demand of DHA needed for adequate organ development, DHA requirement and uptake from the blood is higher in more immature infants, which leads to lower serum DHA levels compared to more mature infants. Further research is needed to investigate this, but our results may demonstrate the need for GA-dependent fatty acid supplementation.
Fatty acids and brain volumes
Even though the importance of DHA for fetal brain development is well established, little is known about the effect of DHA levels on brain development in extremely preterm infants. Two studies, describing different outcomes of the same clinical trial, evaluated the association between early (on average 3 weeks after birth) and near-TEA erythrocyte DHA levels, and early and near-TEA brain development (brain volumes and white matter integrity).14,30 Their findings are in line with our results, showing associations between early erythrocyte DHA levels and cortical gray matter and deep gray matter volumes, and between near-TEA erythrocyte DHA levels and deep gray matter, cerebellar, and brainstem volumes.14 In addition, early erythrocyte DHA levels were associated with lower diffusivity in white matter tracts.30 The similarity of our results underpins the significance of DHA in relation to brain development.
Contrary to our DHA findings, we found no effect of AA levels on brain volumes. AA does not accumulate in the brain during the third trimester of pregnancy as DHA does.1,31 Both AA and DHA are accreted into the fetal brain at high rates, but towards term the DHA fraction increases, whereas AA decreases in the cerebrum.1 This suggests that adequate levels of DHA are more important than AA in extremely preterm infants and may explain why AA levels do not affect brain volumes in our study. Alternatively, it is possible that the DHA:AA ratio, rather than AA levels per se, affects brain development. DHA supplementation in preterm infants reduces AA levels and increases DHA:AA ratio.17 Also in term infants, AA levels decline when they are only supplemented with DHA, and supplementation with a DHA:AA ratio >1 reduces the benefit on cognitive and other functional outcome compared to supplementation with a DHA:AA ratio ≤1.32,33,34 The European Academy of Pediatrics and the Child Health Foundation recommend that all infant formula should be supplemented with both DHA and AA.32
Fatty acids and neurodevelopmental outcome
Most studies that evaluate neurodevelopmental outcome in relation to fatty acids describe interventions with DHA and/or AA supplementation. In a Cochrane systematic review, LCPUFA supplemented formula showed no long-term benefit on visual function, development, and growth in preterm infants,35 but the quality of the evidence was low. Two recent reviews found no effect of enteral DHA supplementation in preterm infants (<37 weeks GA) on behavioral functioning36 and language development37 between 6 months and 10 years of age. However, none of the included studies employed behavior or language development as primary outcome measures, which resulted in relatively small and underpowered studies. In a systematic review on postnatal nutrition to improve brain development,38 three randomized controlled trials on breast milk supplementation with DHA and/or AA showed no effect of a DHA:AA supplementation ratio of 1:1 on cognitive, motor, or behavioral outcome at 8 years of age in preterm born infants,39,40 but a positive effect of DHA:AA supplementation with ratio 1:2 on psychomotor development in preterm born infants at 24 months corrected age.41 In addition, no effect of enteral DHA supplementation on attention was found in preterm born infants at 18 months corrected age,42 nor of parenteral nutrition containing DHA on cognitive, language, or motor scores at 12 and 24 months corrected age.43 On the contrary, another study showed that early (on average 3 weeks after birth) erythrocyte DHA levels were positively associated with motor outcome at 33 months corrected age.30 These studies highlight the complex relationship between fatty acid supplementation, DHA and AA status, and neurodevelopmental outcome, and underline the need for adequately powered, large follow-up studies after intervention trials with fatty acid supplementation.
DHA and GA interplay
Our results show a significant association between DHA levels and GA. In addition, significant brain injury is also associated with GA. It is thus difficult to separate the effect of DHA from the effect of GA on brain volumes. We could not include GA in our final multivariable model, because GA together with DHA resulted in an unstable model. By including DHA and significant brain injury in our model, we have evaluated two important factors that may contribute to brain development in extremely preterm infants. We further analyzed the DHA and GA interplay by creating a DHA curve, and found that infants in the lower 50th percentile had smaller brain volumes, although this did not reach significance. These results suggest a potential causal relationship between DHA AUC and brain volumes, but the study was underpowered to draw final conclusions. We also analyzed the association between GA and brain volumes and found very similar results as with the association between DHA and brain volumes. We therefore speculate that the association between GA and brain volumes may be modified by DHA, but this requires conformation in studies on larger cohorts.
Limitations
Due to low quality (e.g., severe motion artifact, missing T2w images, and field of view not covering the complete brain parenchyma), a large number of MR scans (14/66) was unsuitable for automatic segmentation. The remaining images showed variation with regard to acquisition parameters (as described in the MR image acquisition section). Together with the relatively small number of included infants, this may have resulted in some true effects being missed. Second, in our study cohort, 23% of the infants suffered from significant brain injury, which was more common in the more immature infants. Since DHA levels were lower in the more immature infants, and significant brain injury may affect brain growth, significant brain injury may be a confounder in our analyses. To limit this risk, we included significant brain injury in our statistical models. Finally, although our results suggest a causal relationship between DHA AUC and brain volumes, no causality could be statistically proven due to the small number of included infants.
Future directions
Our results show that maintaining DHA and AA at adequate levels in extremely preterm infants remains a challenge. Two randomized controlled trials are currently evaluating the effect of enteral supplementation with DHA and AA in a DHA:AA ratio of 1:2 in extremely preterm infants from birth until TEA on retinopathy of prematurity (MegaDonnaMega trial, clinicaltrials.gov: NCT03201588) and brain maturation (ImNuT trial, clinicaltrials.gov: NCT03555019). In addition, a more aggressive approach for parenteral supplementation may be required. To gain more insight into the distribution of DHA and AA levels in infants with different GAs and to confirm our results regarding brain volumes, our study should be replicated in a larger study cohort.
Conclusion
Serum levels of DHA, but not AA, during the first 28 postnatal days were positively associated with volumes of several brain structures in extremely preterm infants at TEA. Future research is needed to explore whether fatty acid supplementation after birth can improve brain development in this population.
References
Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 120, S129–S138 (1992).
Mallick, R., Basak, S. & Duttaroy, A. K. Docosahexaenoic acid,22:6n-3: Its roles in the structure and function of the brain. Int. J. Dev. Neurosci. 79, 21–31 (2019).
Lauritzen, L., Hansen, H. S., Jorgensen, M. H. & Michaelsen, K. F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 40, 1–94 (2001).
Hadley, K. B., Ryan, A. S., Forsyth, S., Gautier, S. & Salem, N. Jr. The essentiality of arachidonic acid in infant development. Nutrients 8, 216 (2016).
De Rooy, L., Hamdallah, H. & Dyall, S. C. Extremely preterm infants receiving standard care receive very low levels of arachidonic and docosahexaenoic acids. Clin. Nutr. 36, 1593–1600 (2017).
Lapillonne, A., Eleni dit Trolli, S. & Kermorvant-Duchemin, E. Postnatal docosahexaenoic acid deficiency is an inevitable consequence of current recommendations and practice in preterm infants. Neonatology 98, 397–403 (2010).
Martin, C. R. et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 159, 743–749.e741–742 (2011).
Lapillonne, A. & Jensen, C. L. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot. Essent. Fatty Acids 81, 143–150 (2009).
Baack, M. L., Puumala, S. E., Messier, S. E., Pritchett, D. K. & Harris, W. S. What is the relationship between gestational age and docosahexaenoic acid (DHA) and arachidonic acid (ARA) levels? Prostaglandins Leukot. Essent. Fatty Acids 100, 5–11 (2015).
Lapillonne, A. et al. The use of low-EPA fish oil for long-chain polyunsaturated fatty acid supplementation of preterm infants. Pediatr. Res. 48, 835–841 (2000).
Lapillonne, A., Brossard, N., Claris, O., Reygrobellet, B. & Salle, B. L. Erythrocyte fatty acid composition in term infants fed human milk or a formula enriched with a low eicosapentanoic acid fish oil for 4 months. Eur. J. Pediatr. 159, 49–53 (2000).
Hellstrom, A. et al. Docosahexaenoic acid and arachidonic acid levels are associated with early systemic inflammation in extremely preterm infants. Nutrients 12, 1996 (2020).
Khwaja, O. & Volpe, J. J. Pathogenesis of cerebral white matter injury of prematurity. Arch. Dis. Child Fetal Neonatal Ed. 93, F153–F161 (2008).
Kamino, D. et al. Postnatal polyunsaturated fatty acids associated with larger preterm brain tissue volumes and better outcomes. Pediatr. Res. 83, 93–101 (2018).
Keunen, K. et al. Brain tissue volumes in preterm infants: prematurity, perinatal risk factors and neurodevelopmental outcome: a systematic review. J. Matern. Fetal Neonatal Med. 25(Suppl. 1), 89–100 (2012).
Keunen, K. et al. Brain volumes at term-equivalent age in preterm infants: imaging biomarkers for neurodevelopmental outcome through early school age. J. Pediatr. 172, 88–95 (2016).
Najm, S. et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: a randomized controlled trial. Clin. Nutr. ESPEN 20, 17–23 (2017).
Lofqvist, C. A. et al. Association of retinopathy of prematurity with low levels of arachidonic acid: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 136, 271–277 (2018).
Hansen-Pupp, I. et al. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatr. Res. 69, 448–453 (2011).
Bockmann, K. A. et al. Fatty acid composition of adipose tissue at term indicates deficiency of arachidonic and docosahexaenoic acid and excessive linoleic acid supply in preterm infants. Eur. J. Nutr. 60, 861–872 (2021).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Kidokoro, H., Neil, J. J. & Inder, T. E. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am. J. Neuroradiol. 34, 2208–2214 (2013).
Makropoulos, A. et al. The developing human connectome project: a minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage 173, 88–112 (2018).
Gousias, I. S. et al. Magnetic resonance imaging of the newborn brain: manual segmentation of labelled atlases in term-born and preterm infants. Neuroimage 62, 1499–1509 (2012).
Makropoulos, A. et al. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans. Med. Imaging 33, 1818–1831 (2014).
Niklasson, A. et al. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981). Acta Paediatr. Scand. 80, 756–762 (1991).
Express Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS). Acta Paediatr. 99, 978–992 (2010).
Express Group. et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 301, 2225–2233 (2009).
Baack, M. L., Puumala, S. E., Messier, S. E., Pritchett, D. K. & Harris, W. S. Daily enteral DHA supplementation alleviates deficiency in premature infants. Lipids 51, 423–433 (2016).
Tam, E. W. et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr. Res. 79, 723–730 (2016).
Klevebro, S., Juul, S. E. & Wood, T. R. A more comprehensive approach to the neuroprotective potential of long-chain polyunsaturated fatty acids in preterm infants is needed-should we consider maternal diet and the n-6:n-3 fatty acid ratio? Front. Pediatr. 7, 533 (2019).
Koletzko, B. et al. Should formula for infants provide arachidonic acid along with DHA? A position paper of the European Academy of Paediatrics and the Child Health Foundation. Am. J. Clin. Nutr. 111, 10–16 (2020).
Colombo, J. et al. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am. J. Clin. Nutr. 98, 403–412 (2013).
Colombo, J. et al. acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot. Essent. Fatty Acids 121, 52–56 (2017).
Moon, K., Rao, S. C., Schulzke, S. M., Patole, S. K. & Simmer, K. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst. Rev. 12, CD000375 (2016).
Gould, J. F., Roberts, R. M. & Makrides, M. The influence of omega-3 long-chain polyunsaturated fatty acid, docosahexaenoic acid, on child behavioral functioning: a review of randomized controlled trials of DHA supplementation in pregnancy, the neonatal period and infancy. Nutrients. 13, 415 (2021).
Gawlik, N. R., Anderson, A. J., Makrides, M., Kettler, L. & Gould, J. F. The influence of DHA on language development: a review of randomized controlled trials of DHA supplementation in pregnancy, the neonatal period, and infancy. Nutrients 12, 3106 (2020).
Hortensius, L. M., van Elburg, R. M., Nijboer, C. H., Benders, M. & de Theije, C. G. M. Postnatal nutrition to improve brain development in the preterm infant: a systematic review from bench to bedside. Front. Physiol. 10, 961 (2019).
Almaas, A. N. et al. Long-chain polyunsaturated fatty acids and cognition in VLBW infants at 8 years: an RCT. Pediatrics 135, 972–980 (2015).
Almaas, A. N. et al. Diffusion tensor imaging and behavior in premature infants at 8 years of age, a randomized controlled trial with long-chain polyunsaturated fatty acids. Early Hum. Dev. 95, 41–46 (2016).
Alshweki, A. et al. Effects of different arachidonic acid supplementation on psychomotor development in very preterm infants; a randomized controlled trial. Nutr. J. 14, 101 (2015).
Hewawasam, E. et al. DHA supplementation in infants born preterm and the effect on attention at 18 months’ corrected age: follow-up of a subset of the N3RO randomised controlled trial. Br. J. Nutr. 125, 420–431 (2021).
Thanhaeuser, M. et al. A randomized trial of parenteral nutrition using a mixed lipid emulsion containing fish oil in infants of extremely low birth weight: neurodevelopmental outcome at 12 and 24 months corrected age, a secondary outcome analysis. J. Pediatr. 226, 142–148 (2020).
Acknowledgements
We would like to thank all infants and their parents who participated and all clinical and research staff involved in the clinical trial. This study was supported by the Swedish Medical Research Council #2016-01131, The Gothenburg Medical Society, and Government grants under the ALF agreement ALFGBG-717971, De Blindas Vänner and Knut and Alice Wallenberg Clinical Scholars. This work used computing resources of the UK MEDical BIOinformatics partnership—aggregation, integration, visualization, and analysis of large, complex data (UK MED-BIO), which is supported by the Medical Research Council [grant number MR/L01632X/1]. This work used computing resources provided by the Swedish National Infrastructure for Computing (SNIC) at Chalmers Centre for Computational Science and Engineering (C3SE), partially funded by the Swedish Research Council through grant agreement no. 2018-05973. L.M.H. received funding from the Athena grant, “Utrecht Center for Food and Health—research program specialized nutrition,” subsidy from the Dutch Ministry of Economic Affairs, Utrecht Province and the municipality of Utrecht. I.B.-B. received grants from the Sahlgrenska University Hospital (SU 2018-03591, SU 2018-04164, ALFGBG-925851) and the University of Gothenburg (E2018/478).
Author information
Authors and Affiliations
Contributions
All authors have met the Pediatric Research authorship requirements: substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: L.M.H., W.H., R.A.H., F.G., M.X.A., A.K.N., M.L.T., R.M.v.E., A.H., M.J.N.L.B. Drafting the article or revising it critically for important intellectual content: L.M.H., W.H., K.S., R.A.H., I.M.B.-B., F.G., M.X.A., A.K.N., M.L.T., R.M.v.E., A.H., and M.J.N.L.B. Final approval of the version to be published: L.M.H., W.H., K.S., R.A.H., I.M.B.-B., F.G., M.X.A., A.K.N., M.L.T., R.M.v.E., A.H., and M.J.N.L.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Informed consent from parents or guardians was obtained in writing before the inclusion of the infant in the trial.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hortensius, L.M., Hellström, W., Sävman, K. et al. Serum docosahexaenoic acid levels are associated with brain volumes in extremely preterm born infants. Pediatr Res 90, 1177–1185 (2021). https://doi.org/10.1038/s41390-021-01645-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01645-w
This article is cited by
-
Maternal milk in the NICU: An everyday intervention to improve brain development
Pediatric Research (2024)
-
The influence of nutrition on white matter development in preterm infants: a scoping review
Pediatric Research (2023)
-
Larger brain volumes at term-equivalent age in infants born preterm: an alternative explanation
Pediatric Research (2021)