Abstract
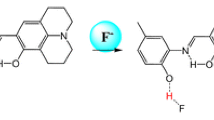
We synthesized an original reversible colorimetric chemosensor PDJ ((E)-9-((2-(6-chloropyridazin-3-yl)hydrazono)methyl)-2,3,6,7-tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-ol) for the detection of F−. PDJ displayed a selective colorimetric detection to F− with a variation of color from colorless to yellow. Limit of detection of PDJ for F− was calculated as 12.1 µM. The binding mode of PDJ and F− turned out to be a 1:1 ratio using Job plot. Sensing process of F− by PDJ was demonstrated by 1H NMR titration and DFT calculation studies that suggested hydrogen bond interactions followed by deprotonation. Moreover, the practicality of PDJ was demonstrated via a reversible test with TFA (trifluoroacetic acid).









Similar content being viewed by others
References
Sahu S, Sikdar Y, Bag R et al (2019) Visual detection of fluoride ion based on ICT mechanism. Spectrochim Acta - Part A Mol Biomol Spectrosc 213:354–360
Gupta N, Singhal D, Singh AK et al (2017) A highly selective chromogenic sensor for Mn2+, turn-off fluorometric for Hg2+ ion, and turn-on fluorogenic sensor for F- ion with the practical application. Spectrochim Acta - Part A Mol Biomol Spectrosc 176:38–46
Singh A, Tom S, Trivedi DR (2018) Aminophenol based colorimetric chemosensor for naked-eye detection of biologically important fluoride and acetate ions in organo-aqueous medium: Effective and simple anion sensors. J Photochem Photobiol A Chem 353:507–520
Lim C, Seo H, Choi JH et al (2018) Highly selective fluorescent probe for switch-on Al3+ detection and switch-off F- detection. J Photochem Photobiol A Chem 356:312–320
Zhang YM, He JX, Zhu W et al (2019) Novel pillar[5]arene-based supramolecular organic framework gel for ultrasensitive response Fe3+ and F– in water. Mater Sci Eng C 100:62–69
Jeong HY, Lee SY, Kim C (2017) Furan and Julolidine-Based “Turn-on” Fluorescence Chemosensor for Detection of F– in a Near-Perfect Aqueous Solution. J Fluoresc 27:1457–1466
Ding S, Xu A, Li M et al (2020) Theoretical study on the sensing mechanism of an ON1-OFF-ON2 type fluoride fluorescent chemosensor. Spectrochim Acta - Part A Mol Biomol Spectrosc 237:118397
Das A, Dighe SU, Das N et al (2019) β-carboline-based turn-on fluorescence chemosensor for quantitative detection of fluoride at PPB level. Spectrochim Acta - Part A Mol Biomol Spectrosc 220:117099
Peng Y, Dong YM, Dong M, Wang YW (2012) A selective, sensitive, colorimetric, and fluorescence probe for relay recognition of fluoride and Cu(II) ions with “off-On-Off” switching in ethanol-water solution. J Org Chem 77:9072–9080
Yadav P, Kumari M, Jain Y et al (2020) Antipyrine based Schiff’s base as a reversible fluorescence turn “off-on-off” chemosensor for sequential recognition of Al3+ and F− ions: A theoretical and experimental perspective. Spectrochim Acta - Part A Mol Biomol Spectrosc 227:117596
Wu N, Zhao LX, Jiang CY et al (2020) A naked-eye visible colorimetric and fluorescent chemosensor for rapid detection of fluoride anions: Implication for toxic fluorine-containing pesticides detection. J Mol Liq 302:112549
Gowri A, Veeraragavan V, Kathiresan M, Kathiravan A (2019) A pyrene based colorimetric chemosensor for CO2 gas detection triggered by fluoride ion. Chem Phys Lett 719:67–71
Karuppiah K, Muniyasamy H, Sepperumal M, Ayyanar S (2020) Design and synthesis of new salicylhydrazone tagged indole derivative for fluorometric sensing of Zn2+ ion and colorimetric sensing of F– ion: Applications in live cell imaging. Microchem J 159:105543
Landge SM, Lazare DY, Freeman C et al (2020) Rationally designed phenanthrene derivatized triazole as a dual chemosensor for fluoride and copper recognition. Spectrochim Acta - Part A Mol Biomol Spectrosc 228:117758
Ma L, Leng T, Wang K et al (2017) A coumarin-based fluorescent and colorimetric chemosensor for rapid detection of fluoride ion. Tetrahedron 73:1306–1310
Fang H, Gan Y, Wang S, Tao T (2018) A selective and colorimetric chemosensor for fluoride based on dimeric azulene boronate ester. Inorg Chem Commun 95:17–21
Dong M, Peng Y, Dong YM et al (2012) A selective, colorimetric, and fluorescent chemodosimeter for relay recognition of fluoride and cyanide anions based on 1,1′-binaphthyl scaffold. Org Lett 14:130–133
Lin Q, Gong GF, Fan YQ et al (2019) Anion induced supramolecular polymerization: A novel approach for the ultrasensitive detection and separation of F–. Chem Commun 55:3247–3250
Rajasekhar K, Narayanaswamy N, Murugan NA et al (2016) A High Affinity Red Fluorescence and Colorimetric Probe for Amyloid β Aggregates. Sci Rep 6:1–10
Goswami S, Hazra A, Chakrabarty R, Fun HK (2009) Recognition of carboxylate anions and carboxylic acids by selenium-based new chromogenic fluorescent sensor: A remarkable fluorescence enhancement of hindered carboxylates. Org Lett 11:4350–4353
Lee HJ, Park SJ, Sin HJ et al (2015) A selective colorimetric chemosensor with an electron-withdrawing group for multi-analytes CN– and F–. New J Chem 39:3900–3907
Beneto AJ, Siva A (2017) A phenanthroimidazole based effective colorimetric chemosensor for copper(II) and fluoride ions. Sens Actuators B Chem 247:526–531
Moon KS, Singh N, Lee GW, Jang DO (2007) Colorimetric anion chemosensor based on 2-aminobenzimidazole: naked-eye detection of biologically important anions. Tetrahedron 63:9106–9111
Anbu Durai W, Ramu A (2020) Hydrazone Based Dual – Responsive Colorimetric and Ratiometric Chemosensor for the Detection of Cu2+/F– Ions: DNA Tracking, Practical Performance in Environmental Samples and Tooth Paste. J Fluoresc 30:275–289
Zabihi FS, Mohammadi A (2020) Synthesis and application of a new chemosensor based on the thiazolylazo-quinazolinone hybrid for detection of F− and S2− in aqueous solutions. Spectrochim Acta - Part A Mol Biomol Spectrosc 238:118439
Chatterjee C, Sethi S, Mukherjee V et al (2020) Triazole derived azo-azomethine dye as a new colorimetric anion chemosensor. Spectrochim Acta - Part A Mol Biomol Spectrosc 226:117566
Shyamaprosad Goswami RC (2012) An imidazole based colorimetric sensor for fluoride anion. Eur J Chem 3:455–460
Wang Q, Xie Y, Ding Y et al (2010) Colorimetric fluoride sensors based on deprotonation of pyrrole-hemiquinone compounds. Chem Commun 46:3669–3671
dos Santos CH, Uchiyama NM, Bagatin IA (2019) Selective azo dye-based colorimetric chemosensor for F−, CH3COO− and PO43−. Spectrochim Acta - Part A Mol Biomol Spectrosc 210:355–361
Li Z, Wang S, Xiao L et al (2018) An efficient colorimetric probe for fluoride ion based on schiff base. Inorg Chim Acta 476:7–11
Zang L, Wei D, Wang S, Jiang S (2012) A phenolic Schiff base for highly selective sensing of fluoride and cyanide via different channels. Tetrahedron 68:636–641
Wang X, Bai T, Chu T (2021) A molecular design for a turn-off NIR fluoride chemosensor. J Mol Model 27:104
Helal A, Thao NTT, Lee SW, Kim HS (2010) Thiazole-based chemosensor II: Synthesis and fluorescence sensing of fluoride ions based on inhibition of ESIPT. J Incl Phenom Macrocycl Chem 66:87–94
Lee JJ, Park GJ, Choi YW et al (2015) Detection of multiple analytes (CN– and F–) based on a simple pyrazine-derived chemosensor in aqueous solution: Experimental and theoretical approaches. Sens Actuators B Chem 207:123–132
Jo TG, Na YJ, Lee JJ et al (2015) A diaminomaleonitrile based selective colorimetric chemosensor for copper(II) and fluoride ions. New J Chem 39:2580–2587
Ganesan JS, Gandhi S, Radhakrishnan K et al (2019) Execution of julolidine based derivative as bifunctional chemosensor for Zn2+ and Cu2+ ions: Applications in bio-imaging and molecular logic gate. Spectrochim Acta - Part A Mol Biomol Spectrosc 219:33–43
Deepa A, Srinivasadesikan V, Lee SL, Padmini V (2020) Highly Selective and Sensitive Colorimetric and Fluorimetric Sensor for Cu2+. J Fluoresc 30:3–10
Yun D, Chae JB, Kim C (2019) A novel benzophenone-based colorimetric chemosensor for detecting Cu2+ and F–. J Chem Sci 131:1–10
Budzák Š, Jacquemin D (2018) Excited state intramolecular proton transfer in julolidine derivatives: An: ab initio study. Phys Chem Chem Phys 20:25031–25038
Ryu HH, Lee YJ, Kim SE et al (2016) A colorimetric F– chemosensor with high selectivity: experimental and theoretical studies. J Incl Phenom Macrocycl Chem 86:111–119
Koçak R, Dastan A (2021) Synthesis of dibenzosuberenone-based novel polycyclic π-conjugated dihydropyridazines, pyridazines and pyrroles. Beilstein J Org Chem 17:719–729
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford CT
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies. Theor Chim Acta 28:213–222
Francl MM, Pietro WJ, Hehre WJ et al (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77:3654–3665
Klamt A, Moya C, Palomar J (2015) A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J Chem Theory Comput 11:4220–4225
Kumari N, Jha S, Bhattacharya S (2011) Colorimetric probes based on anthraimidazolediones for selective sensing of fluoride and cyanide ion via intramolecular charge transfer. J Org Chem 76:8215–8222
Yang R, Li K, Wang K et al (2003) Porphyrin assembly on β-cyclodextrin for selective sensing and detection of a zinc ion based on the dual emission fluorescence ratio. Anal Chem 75:612–621
Olivieri AC (2014) Analytical figures of merit: From univariate to multiway calibration. Chem Rev 114:5358–5378
Acknowledgements
We politely acknowledged National Research Foundation of Korea (2018R1A2B6001686).
Author information
Authors and Affiliations
Contributions
Dongkyun Gil (60% contributions), Boeon Suh (10% contributions), Cheal Kim (30% contributions).
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human or animal subjects.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gil, D., Suh, B. & Kim, C. A New Reversible Colorimetric Chemosensor Based on Julolidine Moiety for Detecting F−. J Fluoresc 31, 1675–1682 (2021). https://doi.org/10.1007/s10895-021-02801-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02801-5