Abstract
Two-dimensional black phosphorus (BP) has layer-dependent band gap, high specific surface area, moderate carrier mobility, excellent photothermal effect, inherent photoacoustic properties, excellent biodegradability and biocompatibility, making it an ideal option for applications in biomedicine such as photothermal therapy, disease diagnosis, biological imaging and so on. Despite of these advantages, the two-dimensional BP has its own obstacle limiting its practical application, such as its instability, and there is no practical method for synthetization of large size BP flakes for industrial application. This review firstly introduces interesting properties of BP such as its biocompatibility, biodegradability, anisotropy, moderate carrier mobility and so on. Then summarizes the preparation methods of two-dimensional BP: non-liquid phase methods (e.g. mechanical peeling and chemical vapor deposition) and liquid phase methods (e.g. liquid-phase exfoliation and wet chemistry methods). The application of BP in the biomedical field such as photoacoustic imaging, fluorescence imaging, photothermal imaging, circulating tumor DNA detection, cancer treatment and treatment of neurodegenerative diseases are introduced later. Then we discussed the current problems of BP and methods to enhance the stability of BP including encapsulation, functionalization, liquid phase environmental protection and doping. Finally, some future trends in the research of two-dimensional BP are envisaged. It is believed that two-dimensional BP will have a broad application prospect in various fields especially in biomedicine due to its biocompatibility and biodegradability.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Since the rediscovery of black phosphorus (BP) as a 2D material in 2014, 2D BP has become a rising star in the 2D material family [1]. As a most stable allotrope among the phosphorus element family [2], the earliest report on BP was published in 1865 [3, 4], the morphology density, thermal stability, hardness and other properties of BP were studied in 1914 [5, 6]. Then the research on BP has been suspended for about a century only few articles were reported on BP.
As the research on two-dimensional materials such as graphene [7–9], hexagonal boron nitride (hBN) [10–13], and transition metal dichalcogenides (TMDCs) [14–17] gain extensive interest in the past decades, people re-discovered BP from the perspective of two-dimensional materials [18–21]. Zhang et al fabricated a thin layer of BP nanosheets with a hole mobility up to 1000 and an adjustable direct band gap [1]. Since then, two dimensional BP has become a promising semiconductor material and are widely applicated in nanoelectronics and nanophotonic devices [22–32].
The band gap of BP become broader when the BP become thinner: from 0.3 eV (multilayer) to 2.0 eV (monolayer) [33, 34]. Compared with other 2D materials, it shows stronger absorption capacity in the ultraviolet to mid-infrared regions with moderate carrier mobility [35, 36]. Due to its outstanding absorption capacity, BP are applicated for generation of pulse laser in the mid-infrared region [37–40], and it has been used as a saturable absorber for solid-state lasers for generation of short pulses [41–45].
The most attractive character of BP is that it has excellent biocompatibility and strong in-vivo biodegradability compared with other 2D nanomaterials, which indicate that BP is suitable for multidisciplinary biomedical applications [46–58].
This review highlights the application of BP in biomedical including photoacoustic imaging (PA), fluorescence (FL) imaging, photothermal imaging, circulating tumor DNA (ctDNA) detection, cancer treatment and treatment of ND. The development of BP is briefly introduced in the first part. Then the preparation method of two-dimensional BP is presented in the second part. The crystal and electronic structure of BP, as well as its conductivity, charge mobility, anisotropy and stability, biocompatibility and biodegradability are summarized in the third part. Various methods to enhance the stability of BP are introduced in the fourth part. The application of BP in the biomedical field such as PA, FL imaging, photothermal imaging, ctDNA detection, cancer treatment and treatment of ND are introduced in the fifth part. The challenge of BP in its application in clinical medical is discussed in the final part.
2. Structure and properties
2.1. Crystal structure
BP has three different crystal structure, orthorhombic phase, hexagonal phase and cubic phase [59].
Orthogonal phase BP has a fold-like layered structure with a lattice constant of a = 3.31 Å, b = 4.38 Å, c = 10.50 Å, and a layer spacing of 5.3 Å [60]. As shown in figure 1, there are two different bond lengths in the orthogonal phase BP: the bond length connecting the phosphorus atoms within the same plane is 2.22 Å, and the bond length connecting the phosphorus atoms between adjacent planes is 2.24 Å. Each phosphorus atom has a lone electron pair on the fourth orbital, so it is surface active [61–63]. Layers of BP are stacked repeatedly with van der Waals (VDW) forces. The spacing between layers are switchable from 3.21 Å to 5.455 Å.
Figure 1. (a) Crystal structure of layered BP, (b) front view, (c) vertical view, (d) interlayer BP structure diagram. (R1 = 2.22 Å, R2 = 2.24 Å, θ1 = 96.300°, θ2 = 102.095°). [35] John Wiley & Sons. [Copyright © 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution imageWhen the pressure is increased to 5 GPa, the BP undergoes a reversible structural transformation. As the VDW bond is easy to shorten with the increased pressure, while the covalent bond is relatively stable, thus lattice compression and interlayer sliding occur with increased pressure, resulting in the transformation from orthogonal phase semiconductor to hexagonal phase semimetal. When the pressure reaches 10 GPa, the bonds between the phosphorus atoms are rebuilt, and the BP finally transformed to a metallic cubic phase [64].
2.2. Electronic structure and band gap
BP is a semiconductor with direct band gap, and can absorb mid-infrared, near-infrared, visible and ultraviolet light with wavelength less than 4133 nm. The band gap could be adjusted from 0.3 to 2 eV with varied thickness [65, 66].
In 1953, Keyes et al measured the electronic structure of bulk BP and found that bulk BP is a direct band gap semiconductor with a band gap of 0.33 eV [67]. In 2014, Tran et al studied the band structure of BP with different layers using the first principles simulation method. The calculation results show that the band gap of monolayered BP is 2.0 eV, and the band gap decreases to 0.3 eV for bulk BP [68]. In 2016, Feng et al measured the band gap of BP nanosheets with different thickness by the absorption spectra [69]. The experimental results show that the band gap of monolayered BP is 1.73 eV, which decreases to 1.15 eV for double-layer BP. The band gap of three-layer BP is 0.83 eV, and switches to 0.35 eV for bulk BP.
The external stress has a significant effect on the band gap of BP, which leads to the transformation of BP from semiconductor phase to metal phase [70–72]. Compared with the zero-band structure of graphene and the narrow band structure (1.2∼1.8 eV) of molybdenum disulfide [73], the band gap distribution of BP covers a broad gap region from 0.3 eV to 2.0 eV. Its direct band gap structure is in favor of excellent comprehensive carrier mobility and outstanding photo-response characteristic from near-infrared to visible region [74], which makes it suitable for application in photocatalyst, photoelectric devices and biomedical [75].
2.3. Conductivity and charge mobility
In 1953, Keyes measured the resistivity of BP to be 1.5 Ω cm, with a hole concentration of  and an electron mobility of
and an electron mobility of  at room temperature [67]. In 1983, Narita measured the resistivity of BP crystal along different orientations at 200 K, and revealed that there is strong anisotropy in BP, with a maximum resistivity of 285 Ω
at room temperature [67]. In 1983, Narita measured the resistivity of BP crystal along different orientations at 200 K, and revealed that there is strong anisotropy in BP, with a maximum resistivity of 285 Ω  and a minimum resistivity of 0.673 Ω
and a minimum resistivity of 0.673 Ω  [76]. In 2014, Zhang et al found that the carrier mobility of BP varied with thickness. The highest hole mobility can reach as high as 1000 cm2 V−1 s−1, with a corresponding thickness of 10 nm [1]. In 1968, Jim et al discovered that the orthorhombic BP could transform to superconducting state at 4.7 K when the pressure is larger than 100 bar [77].
[76]. In 2014, Zhang et al found that the carrier mobility of BP varied with thickness. The highest hole mobility can reach as high as 1000 cm2 V−1 s−1, with a corresponding thickness of 10 nm [1]. In 1968, Jim et al discovered that the orthorhombic BP could transform to superconducting state at 4.7 K when the pressure is larger than 100 bar [77].
2.4. Anisotropy
BP has a high in-plane anisotropy due to the wrinkled structure, especially for the monolayer or few-layer BP. The anisotropy of BP nanosheets are manifested in its electricity, optics, mechanics, phonon, thermodynamics properties [78–80]. The carrier mobility and conductivity in Zigzag (y) direction is about 50% higher than that in Armchair (x) direction, and the hardness in Zigzag (y) direction is 4 times harder than that in Armchair (x) direction [81]. In addition, the optical properties of BP also show obvious anisotropy. The properties of excitons emitted by monolayer BP can be studied by polarization-resolved photoluminescence spectroscopy. As shown in figure 2(a) [82], the polarization-dependent imaging contrast of the transmitted light (IBP/Isub) ratio between the two extreme cases is 1.8. The two pictures at the top of figure 2(a) respectively show the transmission image of a 70 nm BP sheet with the input polarization Along the a-(0°) and b-(90°) axes. Instead, use a low-intensity laser (λ = 635 nm) on the same BP film to collect image contrast at the phase anisotropic output. The orientation of the sample is very sensitive, as shown in figure 2(b). For a 70 nm thick BP slice, this imaging setting can obtain an intensity contrast of about 17 (IBP, 0/IBP, 45). Yue Liu studied its transport properties by theoretical calculation, and concluded that the mobility anisotropy ratio of single-layer BP is 3–4 [83].
Figure 2. Phase anisotropy imaging provides enhanced intensity contrast. (a) The intensity of the transmitted light as a function of the incident polarization angle. The two blue circles represent the sampling area of BP and substrate. (b) Intensity comparison of light passing through the BP sheet based on phase anisotropy imaging. Use the same 0° (90°) direction definition as in (a). Reprinted with permission from [82]. Copyright (2016) American Chemical Society.
Download figure:
Standard image High-resolution image2.5. Stability
2.5.1. Chemical stability
BP is the most stable elemental form in the phosphorus allotrope. Bulk BP can be heated to 400 °C in air without being ignited, and can stay stable for half a year [84]. However, the stability of phosphorus atoms on surface or edge of the BP is poor, and it is easy to degrade in air. The degradation of BP in air is the result of the synergistic effect of light, water and oxygen: the ultraviolet light transforms oxygen to ozone molecules, and its strong oxidation promotes the oxidation process to form phosphorus oxide. Phosphorus oxide has strong hygroscopicity, and it is easy to react with acid gas and water in air, which cause acidification and deliquescence to BP [85, 86]. Therefore, the surface of BP before the oxidation decomposition process is smooth and hydrophobic, while after oxidation, it becomes hydrophilic [87]. The volume of the two-dimensional BP on the surface layer increases due to the absorption of water in air. Phosphorus atoms on the surface are corroded firstly, and then the corrosion spots appear on the surface, and gradually phosphorus atoms in the inner layer are corrodes [88], leading to the final degradation of BP [89]. This is an important factor restricting the application of BP.
2.5.2. Thermal stability
The thermal stability of BP is poor. The bulk BP begins to decompose at 330 °C in argon, and begins to absorb oxygen at 210 °C in air [90]. The thermal decomposition of BP occurs at 400 °C in vacuum [91], so the BP is not suitable for application in high temperature, which is another bottleneck for its application.
2.6. Biocompatibility and biodegradability
When BP crystals are transformed into nanomaterials, the specific surface area is further expanded, and can load drug examples or nanoparticles [92]. When transformed to nanomaterials, the absorption edge of BP switched from 620 nm to 4130 nm, so it can absorb near-infrared light for generation of photothermal effect, the BP nanomaterials shows excellent photothermal conversion efficiency [93, 94]. As phosphorus is a high content element in human body, BP can directly metabolize into non-toxic phosphate ions, therefore it shows outstanding biocompatibility and biodegradability, and may be applied in the biological field.
3. Preparation of BPs
The fabrication method of ultra-thin BP can be divided into non-liquid phase methods (e.g. mechanical peeling and chemical vapor deposition (CVD)) and liquid phase methods (e.g. liquid-phase exfoliation and wet chemistry methods.) Synthetic methods for ultrathin BP are summarized in the following part:
3.1. Mechanical peeling
The main methods to obtain high-quality two-dimensional materials is mechanical exfoliation, which was proposed by Noveselov and Geim for preparation of graphene [95]. Mechanical exfoliation was firstly applied for preparation of high-quality few-layer BP and is still the most commonly used method up to now [96–99]. The principle of mechanical exfoliation method is that: for two-dimensional material, the adjacent layers are usually combined by VDW forces and are easily exfoliated by mechanical forces. Theoretical calculations show that the layer spacing of BP is larger than that of the graphite, and the VDW force between adjacent layers is thus smaller than graphene, making BP easier to be mechanically exfoliated than graphene. However, compared with graphene, the few-layer BP prepared by the mechanical exfoliation method is usually smaller in size, and it is difficult to obtain samples more than 10 μm [100, 101]. Luo et al prepared a 16.1 nm thick BP flakes using the mechanical peeling method as shown in figure 3 [102].
Figure 3. Scanning electron microscopy image and optical image of the BP flake. Scale bars, 5 μm [102]. Reproduced from [102]. CC BY 4.0.
Download figure:
Standard image High-resolution imageUnlike other two-dimensional materials, the phosphorus atoms of BP are not arranged on the same plane, they form a puckered honeycomb lattice, and its fold-like morphology makes the sample easy to be broken during the exfoliation process. The second characteristic of BP is that the adhesion between BP and the SiO2/Si substrate is weaker when compared with graphene. Thus, the mechanical exfoliation process of BP is generally improved by peeling the BP to a layer of PDMS substrate firstly and then transferring it to the target substrate. This process increases the probability to obtain BP nanosheets with larger size [103, 104]. Plasma etching is introduced to reduce the thickness of the exfoliated samples to obtain few-layer BP [105–109].
Although BP prepared by the mechanical exfoliation method has the advantages of good crystallinity and high quality, the randomness of the thickness and size of the obtained samples undoubtedly limits the application of BP. This method is currently only suitable for usage in laboratories.
3.2. Liquid phase exfoliation
Liquid phase exfoliation method refers to the method to suspend the bulk material in organic solvent that matches the interaction energy of the material, and crush the crystals with ultrasonic energy to obtain two-dimensional material [110–114]. The solubility parameter can partially predict the dispersion of the layered materials in solution. The Hansen solubility parameter (HSP) can be used to predict the exfoliation and dispersion of layered materials in solution. Generally, there are three HSP parameters used to describe the characteristic solute-solvent interaction of a solution or material:  , which represent the dispersion power, polarity and hydrogen bond solubility respectively. For simplicity, we can use the HSP distance
, which represent the dispersion power, polarity and hydrogen bond solubility respectively. For simplicity, we can use the HSP distance  to find an effective solvent for 2D material dispersion. The
to find an effective solvent for 2D material dispersion. The  value is determined by the following formula:
value is determined by the following formula:
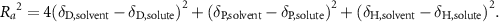
The expected solubility is inversely proportional to the  value [115, 116].
value [115, 116].
The advantage of the liquid phase exfoliation method is that it is easy to obtain a large number of BP nanosheets with a size of about 1 µm, so it has potential application prospects in industry. There are basically three steps for liquid phase exfoliation as shown in figure 4: the target material is dispersed in the corresponding solvent firstly, then ultrasound energy is used to separate the material, finally the sample are centrifuged for collection of the target product [117].
Figure 4. Liquid exfoliation mechanism [117]. Reproduced from [117] with permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageIn 2014, Brent et al used N-methyl-2-pyrrolidone (NMP) as a solvent to exfoliate buck BP, 200 × 200 nm BP nanosheets with three to five layers are obtained. Monolayer and bilayer BP nanosheets with a size of 20 × 20 nm were successfully prepared when extend the exfoliation time [118]. Yasaei et al prepared monolayer BP nanoparticles with good stability and excellent transport performance using stable and polar dimethylformamide and dimethyl sulfoxide as solvents [119]. Hanlon et al prepared stable BP nanosheets with different sizes and thicknesses, which are exfoliated in N-cyclohexyl-2-pyrrolidone solution [120]. Guo et al prepared BP with controllable layers and sizes using mixed solution of NMP as the exfoliation solvent [121]. Zhao et al took a different approach and prepared a high-concentration and stable dispersion of BP nanosheets by exfoliating it in an ionic liquid [122]. In 2020, Xu et al prepares functionalized multilayer BP nanosheets by using succinic acid or L-ascorbic acid assisted tip ultrasonic peeling, and obtained 4–5 layers of BP nanosheets with a thickness of about 2–2.5 nm, the chronoamperometic measurements results show that BP nanosheets have excellent electrocatalytic stability [123].
Generally speaking, the liquid phase exfoliation method can produce a large number of homogeneous BP nanosheets, but it needs ultra-long ultrasonic time, and the obtained BP nanosheets is usually small in size and tends to have some solvent residues.
3.3. CVD
CVD is a method using volatile precursors to react or decompose at high temperatures for generation of two-dimensional materials on the surface of the substrate [124, 125]. So far, CVD has been successfully used to prepare many 2D nanomaterials, including graphene, hBN, TMDCs and so on.
In 2015, Li et al used flexible materials as substrates to achieve large-scale BP film. They deposited red phosphorus (RP) on a flexible polyester substrate using thermal deposition for the formation of a thin RP film firstly, then put the deposited RP film into a 400 °C high-pressure chamber for pressure conversion, and made BP nanosheets finally. The prepared wafer-type BP film has a size of 4 mm and a thickness of about 40 nm. The thickness of the BP film could be adjusted by changing the holding time at 400 °C, and the size of the BP film could be modified by depositing BP on substrates with different size [126]. Smith's et al prepared BP nanosheets by CVD. Firstly, the RP is heated at 600 °C to form an amorphous RP film on the substrate, then the substrate covered with the RP film, the mineralizer tin and tin iodide are heated to 90 °C at a pressure of 27.2 atm, BP nanosheets with large area (0.35–100  ) and variable thickness (3.4–600 nm) were finally produced [127]. In 2018, Li et al used sapphire as a substrate for the synthetization of a high-quality sub-centimeter BP film using RP as precursor at 700 °C and 1.5 GPa as shown in the figure 5. The synthesized BP film are characterized by the Raman mapping and infrared extinction measurement, which indicate that the obtained BP film have a uniform crystal orientation [128].
) and variable thickness (3.4–600 nm) were finally produced [127]. In 2018, Li et al used sapphire as a substrate for the synthetization of a high-quality sub-centimeter BP film using RP as precursor at 700 °C and 1.5 GPa as shown in the figure 5. The synthesized BP film are characterized by the Raman mapping and infrared extinction measurement, which indicate that the obtained BP film have a uniform crystal orientation [128].
Figure 5. Chemical vapor deposition for synthesis of BP film. (a) Synthesis process of BP film. (b) Reaction device diagram for synthesis of BP film on a sapphire disc. (c) Schematic diagram of the influence of pressure and temperature on the conversion of RP to BP. [128] John Wiley & Sons. [© 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution image3.4. Wet chemical method
Wet chemical method refers to a group of solution-based methods including hydrothermal method, solvothermal method, template synthesis method and so on.
In 2016, solvothermal method are used for the preparation of BP nanosheets with industrial RP powder as the precursor and ethanol as the solvent at 400 °C. The prepared nanosheets is about 1 µm in sized and 0.5–4 nm in thickness [129]. Gang et al found that ammonium fluoride can reduce the surface activation energy of layered materials. Therefore, a thinner BP nanosheet layer was prepared by adding a certain amount of ammonium fluoride in the hydrothermal process [130]. In 2018, Tian et al prepared BP nanosheets by solvothermal method. They use white phosphorus as raw material and ethylenediamine as solvent. When adjusted the reaction temperature, BP nanosheets are obtained with different thicknesses. These simple solvothermal methods can be used for production of large-scale BP nanosheets, and can greatly reduce the cost in preparation of BP nanosheets. However, the crystallinity of the BP nanosheets prepared by these methods is still poor and is incomparable with the mechanical exfoliated BP nanosheets [131]. In 2019, Fan et al used two electron-deficient reagents 2, 2, 6, 6-tetramethylpiperidinyl-N-oxyl and triphenylcarbon tetrafluoroborium ([Ph3C] BF4) in organic solvents for the thinning and/or passivation of BP [132]. Field-effect transistors and photoelectric detection devices are constructed using this chemically modified BP sheet, the results show that it can stay stable for up to four months. This method enhanced the stability of BP for industrial application.
4. Biomedical appliances
The application of BP in biomedicine are focused on two parts: medical diagnosis and medical treatment.
4.1. Medical diagnosis
4.1.1. PA
PA is a combination of optical imaging and ultrasound imaging to provide high-contrast and high-resolution tissue imaging [133]. The principle of PA is that when laser irradiate on the nano-medicine, the nano-medicine absorbs light energy and generates thermal expansion [134]. It is worth to mention that the thermal expansion is accompanied by ultrasonic waves, and ultrasound imaging based on ultrasonic signal of nano drugs could be used for in-vivo imaging. When the BP nanoflake is reduced to quantum dots of about 10 nm in size, it shows interesting optical properties and can be used for biological imaging [135, 136].
In 2016, Sun et al prepared BP nanoparticles by solvent-free high-energy mechanical milling (HEMM) technology using RP as raw material. They found that the intensity of the PA signal is linearly proportional to the concentration of nanoparticles [137]. As shown in figure 6, after intravenous injection of BP nanoparticles, the signal intensity retained by the tumor was higher than that of the liver and kidney. This indicates that more BP nanoparticles are tends to retained in the tumor compared with retained in the liver or kidney.
Figure 6. Photoacoustic imaging and photothermal therapy. (a) Schematic diagram of photoacoustic imaging and photothermal therapy. (b) After intravenous injection of PEGylated BP nanoparticles, in vitro photoacoustic images of PEGylated BP nanoparticles solution and in vivo photoacoustic images of liver, kidney and tumor [137]. Reprinted from [137], Copyright (2016), with permission from Elsevier.
Download figure:
Standard image High-resolution imageBecause of its poor stability and degradability in water and air, the optical properties and biological applications of BP are greatly limited. In order to overcome these problems, Sun et al prepared TIL4@BPQDs by mixing BP quantum dots (BPQDs) and titanium sulfonate ligand TIL4 (TIL4), which is synthesized by the reaction of titanium tetraisopropoxide [Ti(OiPr)4] and p-toluene sulfonic acid in N-methyl-2-pyrrolidone. Compared with pure BPQD, TIL4@BPQDs has higher stability in water, which is conducive to further application of BP in biomedicine. Til4@BPQDs shows the best PA signal at 680 nm as shown in figure 7(a). Til4@BPQDs has excellent in-vitro and in-vivo PA effect, which indicates that TIL4@BPQDs can be used in cancer diagnosis [138].
Figure 7. Practical application renderings of PA imaging. (a) PA image of MCF-7 cells in BALB/c nude mouse xenograft tumor after intravenous injection of TiL4@BPQDs, the signal strength bar present on the right. [138] John Wiley & Sons. [© 2017 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim]. (b) Thermal imaging camera of mice injected with BPNS@TA-Mn [139]. Reproduced from [139] with permission of The Royal Society of Chemistry. (c) BP Ve–Ag+ NIR laser-mediated NIR-II PA imaging in mice (1300 nm) [141]. John Wiley & Sons. [© 2020 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution imageIn 2019, Guo et al prepared BPNSs@TA-Mn nanocomposites with excellent stability by coating a layer of tannic acid (TA)-Mn2+ chelate networks on the surface of BP nanosheets (BPNSs). The prepared BPNSs@TA-Mn has excellent photothermal effect and can be used for solid tumor ablation; and it shows high contrast imaging in MRI and PA as shown in figure 7(b), which confirmed that it is suitable for application in medical diagnosis. This BPNS@TA-Mn nanoplatform integrate dual-modal imaging of the excellent magnetic resonance and PA, high photothermal conversion efficiency is realized. Therefore, it should have great potential in imaging-guided interventional photothermal therapy (PTT) [139].
In August 2020, Zeng et al reported a new type of treatment strategy: combining photoacoustic (PA) therapy based on auxiliary BP nanoparticles with checkpoint blockade immunotherapy. Using mitochondrial targeted nanoparticles, PA therapy can cause mechanical damage to mitochondria through PA cavitation, which is a kind of localized damage, thus can eradicate the primary tumor accurately. PA therapy can also be used for generation of tumor associated antigens through the presence of nanoparticles containing R848 as adjuvant, thus promoting a strong anti-tumor immune response. When combinate with checkpoint blockers using anti cytotoxic T lymphocyte antigen 4, the immune response will further promote the infiltration of CD8 and CD4 T cells and increase the radiation of CD8/Foxp3 T cells, thereby inhibiting the growth of distant tumors. In addition, memory T cells detected in the spleen can inhibit tumor recurrence by increasing its number. This method can simultaneously eradicate primary tumor [140].
In order to develop an effective anastomotic nanoparticle with NIR-II PA imaging, Li et al constructed BPQDs vesicles to chelate and release Ag+ for the synergetic photodynamic/Ag+ therapy for the monition and inhibition of tumor growth. The theoretical simulation results show that the embedded Ag+ reduces the band gap of BPQD through strong charge coupling, thus enhance the optical absorption, thus the NIR-II PA signal becomes stronger as shown in figure 7(c). Under the guidance of NIR-II PA bioimaging, the hidden Ag+ can be accurately released with the decomposition of Ve and captured by macrophages located in the diseased area during photodynamic, which is the mechanism of the coordinated cancer photodynamic/Ag+ immunotherapy. BP Ve–Ag+ can be used for monition of pathogenic bacteria through NIR-II PA imaging and for acceleration of wound healing [141].
4.1.2. Fluorescence imaging
BP shows thickness-dependent FL property. When excited, the thinner BP flakes show a strong emission peak at 775 nm, while the thicker BP flakes do not show obvious emission peak [142–146].
Lee et al used an modified liquid phase exfoliation method for preparation of fluorescent BP nanodots with an average diameter of 10 nm. The degradation life of BP nanodots ionized in phosphorus buffered saline (PBS) buffer solution was determined (about 80% of 1.5  BP-nanodot crystals were degraded within ten days). The BP nanocrystals has little or no cytotoxic effect in-vitro, and the BP nanoliposomes are used as biofluorescent agents in medical applications as shown in figure 8(c) [147].
BP-nanodot crystals were degraded within ten days). The BP nanocrystals has little or no cytotoxic effect in-vitro, and the BP nanoliposomes are used as biofluorescent agents in medical applications as shown in figure 8(c) [147].
Figure 8. Application examples of fluorescence imaging. (a) In-vivo NIR-II fluorescence images of mice at different time intervals with intravenous injection of BP@lipid-PEG nanosphere aqueous solution, 1400 nm filter are used for imaging [148]. Reprinted with permission from [148]. Copyright (2019) American Chemical Society. (b) Cellular bioimaging of BP nanoparticles. Confocal microscope image of live HeLa cells incubated with BP-nanodots suspension for 12 h. (a), (d) Bright field (BF) and (b), (e) fluorescence (FL), and merged (c), (f) images. A 30 mW argon laser of (b) 358 nm and (e) 488 nm was used as the excitation source to detect BP-nanodots fluorescence. [147] John Wiley & Sons. [© 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim]. (c) Visual FL bioimaging of soybean sprouts, glass fish, freshwater prawns and lunar jellyfish incubated with Zn@BPQDs for different times (2, 4, 8, 10 days), excited by 365 nm ultraviolet lamp (output power is 6 W) [152]. Reprinted from [152], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageIn September 2018, Meng et al prepared BP nanoparticles (BPNPs) from micro-BP, which has a strong photoluminescence emission at 1.8 eV, and it is used for intensity-based FL imaging. The stability and dispersibility of BPNPs can be improved by coating with silica, the silica coating can also extend the photoluminescence lifetime (PLT) of BPNPs. In this way, the imaging quality of FL lifetime imaging can be improved by using BPNPs @ mSiO2. BPNP's PLT is sensitive to changes in ionic strength, pH, and protein levels, and mSiO2 can easily be functionalized by drugs, proteins, antibodies and other molecules. Therefore, BPNP @ mSiO2 is expected to be integrated into multiple treatment modes. Such as PTT, photodynamic therapy and chemotherapy. Generally speaking, BPNPs are unique materials with multiple functions and have great potential in live cell imaging, cell environment detection and multimodal therapy [149].
Hu et al developed a new FL method for sensitive detection of protease [150]. In this method, BP and its derivatives were used as quenchants and fluorescent probes respectively. The results show that the aqueous solution of cationic probe 1 (perylene probe) [151] shows strong FL emission. Therefore, when the negative charged BP is introduced, probe 1 and BP are bound together by electrostatic interaction, accompanied by strong FL quenching. However, the strong competitive interaction between histones and BP leads to the separation of probe 1 and BP, so the FL peak appeared again. The presence of protease can hydrolyze the histones into small fragments, thereby weakening the interaction between histones and BP, and probe 1 is again quenched and absorbed by BP FL. Therefore, it enables us to monitor protease activity and screen protease inhibitors by detecting the change of FL signal of probe 1. This method has the advantages of label-free, high sensitivity and selectivity, so it expands the application of BP in biological analysis and biomedical related fields.
In June 2019, Xu et al prepared BP@lipid-PEG nanospheres which is composed of dozens of cholesterol-modified BP nanoparticles with an average size of 20 nm, and are used for NIR-II FL imaging in nude mice as shown in figure 8(a). When the wavelength of the emitted light is longer than 1400 nm, blood vessels, liver and spleen can be clearly observed with high signal-to-noise ratio. BP@lipid-polyethylene glycol nanospheres are also used for in-vivo PA imaging to semi-quantitate its biodistribution and pharmacokinetics. Both near-infrared FL imaging and polymerase chain reaction imaging clearly show that blood pressure-lipid-polyethylene glycol nanospheres are gradually metabolized by the liver within 48 h. These results show that the biodegradability and biocompatibility of BP@lipid-polyethylene glycol nanospheres are excellent, and has great potential for applications in near-infrared FL bioimaging [148].
In 2020, Jiang et al prepared Zn@BPQDs through sonication-assisted liquid-phase exfoliation of bulk BP crystals in the presence of Zn ions [152]. Pure BPQDs are synthesized by solvothermal reaction of exfoliated few-layer BP. The morphology, microstructure and spectrum of the prepared Zn@BPQDs and BPQDs were characterized and systematically compared, which showed that the surface coordination of zinc and BP can protect the BP structure from degradation and avoid the oxidation of Zn@BPQDs when the environment is exposed to air and water. The colloid and FL stability of Zn@BPQD is better than BPQD. In order to explore the application prospect of biological detection, Zn@BPQDs and CoOOH nanosheets were combined to construct a new type of GSH FL probe. The probe can react highly sensitive and selective to GSH, and realizes the detection of GSH visible to naked eye as shown in figure 8(c). Therefore, the Zn@BPQD can be used as an effective FL reagent for long-term tracking and in-vitro and in-vivo bioimaging applications. Due to its excellent stability and bright FL, Zn@BPQD has broad application prospects in multifunctional drug delivery carriers, and can accurately diagnose and treat malignant diseases and cancer clinically.
In summary, BP nanodots have excellent biocompatibility, low cytotoxicity, and spontaneous degradation ability, which indicates that they have excellent potential in biomedical applications such as bioimaging and cell tracking.
4.1.3. Photothermal imaging
Photothermal (PT) imaging provide non-FL imaging through the photothermal effect. Firstly, the target is optically excited at the pump wavelength, and then the change in the refractive index of the probe beam is detected, thus non-FL imaging is realized [153].
BP can be used for photothermal imaging with infrared thermal imaging cameras. When the mouse tumor was injected with BPQDs/PLGA nanospheres, the temperature rose rapidly from 32.5 °C to 58.5 °C within 10 min, while the temperature in PBS only increased by 6.2 °C. Sun et al prepared PEGylated BP nanoparticles through improved mechanical grinding technology. This study showed that within 5 min of irradiation by 808 nm laser, the tumor temperature rapidly increased by 25 °C [137]. Zhao et al use Nile blue (NB) dye to modify BPs. Dye-modified BPs (named NB@BPs) have good biocompatibility and show strong PTT and NIR imaging efficiency. In vivo experiments show that NB@BPs can mark tumor sites with red FL and effectively ablate tumors under near-infrared radiation [154].
In 2018, Deng et al prepared 20 nm BP-DEX/PEI (DEX refers to dextran and PEI refers to branched poly(ethyleneimine)) nanoparticles by a solvent-free one-pot HEMM method, and then folic acid (FA) and cyanine7 (Cy7) were functionalized by covalent bonding [155]. The resulting BP-DEX/PEI-FA/Cy7 small nanoparticles have excellent water solubility, colloidal stability, biocompatibility and high photothermal conversion efficiency, and can be used for in-vivo targeted imaging (PA, NIRF) and cancer PTT. Compared with BP nanoparticles without FA, tumor images obtained from BP nanoparticles combined with FA have better brightness and higher intensity as shown in figure 9(b). The treatment results also show that the newly synthesized nanoparticles have high stability and have obvious effects of killing cancer cells.
Figure 9. Application examples of PT imaging. (a) Infrared thermal image of mice bearing 4T1 tumors injected with BP-DEX/PEI-FA/Cy7 solution or PBS, irradiated by 808 nm NIR laser (1.5 W cm−2, 6 min) [155]. Reprinted from [155], Copyright (2018), with permission from Elsevier. (b) The thermal images of mice bearing 4T1 tumor injected with PBS (column 1) or pegylated BP nanoparticles (column 2) respectively, irradiated by 808 nm laser (2.0 W cm−2, 5 min) [137]. Reprinted from [137], Copyright (2016), with permission from Elsevier.
Download figure:
Standard image High-resolution imageThese multifunctional BP nanoparticles are expected to have broad application prospects in cancer diagnosis and treatment.
4.1.4. ctDNA detection
ctDNA is the DNA fragments that fall from the cell ruptures of the primary tumor or even new tumors formed by metastasis [156]. Therefore, it appears in the early stage of tumor, early tumors can be detected by detection of ctDNA [157, 158]. However, effective and practical means to analyze ctDNA is still lacking [159].
In 2020, nitrobenzene functionalized BP nanosheets (NP-BPs) biosensors is used for sensitive and selective detection of ctDNA [160]. The prepared biosensor is shown in figure 10, with a detection limit of 50 fM and a linear detection range of 50 fM–80 pM, which can provide reliable readings in a short time (15 min). In addition, NP-BP-based biosensors can be used for distinguish of single nucleotide polymorphisms in clinical serum samples. It is foreseeable that the sensor platform based on NP-BPs has great potential in early cancer diagnosis and monitoring of cancer development.
Figure 10. Schematic diagram of NP-BPs-based biosensor for ctDNA detection based on ssDNA/dsDNA recognition capability. The fluorescent dye is FAM [160]. Reprinted from [160], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution image4.2. Medical treatment
4.2.1. Cancer treatment
In September 2015, Yu et al published a liquid stripping method for combined probe of ultrasound and water. Ultrasound was used to control the preparation of ultra-small BPQD with a dimension of about 2.6 nm [161]. The BPQD is demonstrated to have excellent near-infrared optical properties at 808 nm: the extinction coefficient is 14.8  , and the photothermal conversion efficiency is 28.4%. Under the irradiation of near-infrared laser, it can significantly kill tumor cells and show good biocompatibility in many cell lines. Furthermore, as phosphorus is an essential element in organism, its application in cancer treatment has incomparable advantages.
, and the photothermal conversion efficiency is 28.4%. Under the irradiation of near-infrared laser, it can significantly kill tumor cells and show good biocompatibility in many cell lines. Furthermore, as phosphorus is an essential element in organism, its application in cancer treatment has incomparable advantages.
In October 2016, Yu et al further published a biodegradable photothermal conversion material based on BP, for efficient and safe tumor photothermal treatment [162]. An emulsification solvent volatilization method is used for preparation of a core–shell structured nanosphere (BPQDs/PLGA) with a polymer (PLGA) encapsulated BPQDs. The formed polymer shell layer can make the internal BPQDs isolated from the physiological environment to ensure the stable performance of the BPQDs during the treatment process. When the photothermal treatment is finished, the BPQDs will be slowly released and degraded as the PLGA shell gradually degrades, and then safely metabolized out of the body. Experiments showed that BPQDs/PLGA has good biological safety and passive tumor targeting, and exhibits high photothermal treatment efficiency as shown in figure 11. Five minutes irradiation of near-infrared light can effectively kill tumors. The new type of biodegradable photothermal conversion material can promote the actual clinical application of photothermal treatment technology.
Figure 11. Analysis of pharmacokinetic and biodistribution in tumor photothermal treatment by BP. (a) Blood circulation curve of BPQDs/PLGA NSs labeled with Cy5.5 (a commonly used NIR fluorescent dye). (b) In-vivo fluorescence images of mice were processed by NSs. (c) In vitro fluorescence images of tumor and various organs 24 h after NSs treatment. (d) Fluorescence microscopy images of tumor sections from NSs treated mice. DAPI staining showed NSs in red and nuclei in blue. (Scale bar: upper row 1 mm, lower row 50 μm). (e) Quantitative biodistribution analysis of NSs in mice. Reproduced from [162]. CC BY 4.0.
Download figure:
Standard image High-resolution imageIn November 2016, Liu et al made important progress in the multi-modal treatment of tumors [163]. They prepared negative charged BP nanosheets by ultrasonic exfoliation using their multi-fold structure. The loading of doxorubicin (DOX) is as high as 950%, which is much higher than the loading of nano-drug carrier. When excited by near-infrared light, the nanosheets can absorb light effectively for generation of heat and reactive oxygen species (ROS), thereby accelerate the release of DOX, and increase the permeability of cell membranes and the uptake of drugs. In-vitro and in-vivo experiments have showed that photothermal and photodynamic activity of BP, together with the chemotherapy of Adriamycin, the photothermal and photodynamic activity of BP, form a multimode treatment to eliminate tumors. BP can be gradually decomposed into harmless phosphate ions in the body. The results of blood routine analysis and liver and kidney function tests show that the biochemical indicators of the rats injected with BP are harmless. Based on this, the BP nano-drug delivery system provides a brand-new platform for realization of safe and efficient treatment for tumors.
In October 2016, Zhang et al developed a BP nanosheet carrier system with the clinical chemotherapy drug DOX loaded [164]. Due to the easy-for-functionalization of the BP nanoflake and the large specific surface area, it builds a foundation for the large amount of adsorption of chemotherapeutic drug molecules. The excellent optical and photothermal conversion properties enable it to generate localized high heat under 808 nm laser irradiation. The photothermal treatment of tumors can also drive the release of drugs. Research on the safety of BP drug-loaded nanosheets in-vivo shows that BP drug-loaded nanosheets have excellent biocompatibility. The biological response-adjusted chemotherapy-PTT combined treatment used in the study has achieved enhanced tumor suppression effects in immunodeficient nude mice. This research provides new ideas for the application of BP in biomedicine.
In 2017, Yang et al reported a multifunctional BPs @ Au @ Fe3O4 for plasma photothermal treatment based on the new photosensitizer (PS) BP, which are functionalized with Au nanoparticles and Fe3O4 nanoparticles in a gentle and reproducible way [165]. The resulting product is analyzed to verify its feasibility in biological applications. Chemical and structural evidence showed that the prepared BPs @ Au @ Fe3O4 platform has excellent biocompatibility, high stability under physiological conditions, wide light absorption band and photodegradation under near-infrared irradiation characteristic. It is impressive that the platform's high in-vivo and in-vitro anti-tumor efficiency has been achieved. In summary, BPs @ Au @ Fe3O4 may have been used as a carrier for effective treatment for cancer.
In October 2018, Liu et al synthesized biodegradable BP nanosheets to mediate the specific delivery of human telomerase reverse transcriptase (hTERT) and small interfering RNA (siRNA) for synergistical treatment of cancer [166]. Polyethylene glycol (PEG) and polyethyleneimine (PEI) dual-functionalized BP nanosheets (PPBP) and siRNA are used for the collaborative treatment of cancer. PEG modified BP not only improve the biocompatibility of BP but also enhance its physiological stability. The PPBP nanosheets modified with positively charged PEI can increase the efficiency of loading siRNA and enhance the uptake ability of cells. PPBP nanosheets showed both photodynamic therapy (PDT) and PTT activities when radiated by laser with different wavelength as shown in figure 12. Specifically, PPBP lacks stability in acidic and high ROS surroundings, so siRNA can target cancer cells through the specific degradation of PPBP, and is mediated by acid lysosomes and local light-induced ROS production. In addition, PEI-functionalized PPBP allows siRNA to escape from the lysosome, thereby targeted delivery to the cytoplasm of cancer cells. In a word, hTERT siRNA loaded PPBP significantly inhibited tumor growth and metastasis. The PEG and PEI dual-functionalized BP nanosheets reported in this work may also provide a new and promising way to provide other siRNAs for cancer targeted gene therapy.
Figure 12. Schematic diagram of biodegradable BP nanosheets mediating the specific delivery of hTERT siRNA for collaborative cancer treatment. Reprinted with permission from [166]. Copyright (2019) American Chemical Society.
Download figure:
Standard image High-resolution imageIn August 2019, Wang et al prepared a new BP composite material: firstly, the chemotherapeutic agent docetaxel (DTX) was coupled with BPQDs, and then the BPQDs were loaded into polylactic-co-glycolic acid copolymer (PLGA), thus the multifunctional nanoagent BP/DTX@PLGA is finally prepared [166]. Docetaxel (DTX) is targeted for primary tumors and lung metastatic tumors, and it shows obvious chemothermal properties, which helps to inhibit the growth of metastatic tumors. Therefore, BP/DTX@PLGA has the tropism to target both primary tumors and lung metastatic tumors. In this way, fine-tuning combined chemo-PTT can be used to increase the temperature through NIR laser radiation to eliminate lung metastatic tumors. On the other hand, mechanism studies have found that DTX in the nanocomposite will be released quickly during the heating process and heating treatment process, which will also induce the apoptosis-dependent cell death. This research introduce a new type of multifunctional nanomedicine that can effectively treat cancer metastasis.
In November 2019, Yang et al combined chlorin e6 (Ce6) PS with BP@PEG NSs (PEG-NH2-modified BP nanosheets) to construct a new dual-mode imaging delivery platform, which not only shows good biocompatibility, but also shows PTT/PDT effect in cancer treatment [167]. The photothermal conversion efficiency (PCE) of this new platform is as high as 43.6%, which is much higher than that of BP@PEG NSS (28.7%). In-vivo experiments confirmed that BP@PEG/CE6 NSS also had better tumor targeting properties. In-vitro experiments revealed the mechanism of PTT/PDT effect of BP@PEG/CE6 NSS: when CE6 was released from the platform, it would significantly produce ROS, and ROS would activate transcription factors through concentration regulation, and then kill cancer cells through signal transduction within cells. In general, both in-vivo and in-vitro experiments showed that the combination of BP@PEG-NSS and CE6 can significantly improve its ability to kill tumor cells, which provides great potential for clinical effective PTT/PDT combination therapy guided by FL/infrared imaging, and provides possibilities for further application of multifunctional nano medical platform.
In April 2020, Deblin et al developed a multifunctional nano-conjugate (BP-CuS-FA), which integrate BP nanosheets with plasma CuS nanoparticles, and then target partial FA-PEG-NH2 in a mild and direct process. The results show that BP-CuS-FA shows photodegradation. It is a single wavelength near-infrared light activated collaborative PDT-PTT process, with preferable biocompatibility. The growth rate of tumor was significantly inhibited when treated with BP-CuS-FA with combined PDT-PTT effect. At the same time, folate receptor actively promotes the accumulation of BP-CuS-FA in tumor. After internalization, the nanocomposites will produce heat energy and reactive oxygen species under the irradiation of near-infrared light, which can induce the apoptosis of cancer cells and the destruction of endosomes. Ingeniously, BP nanoparticles degrade ROS generated by oxidation process, thus reducing the side effects in the treatment process. BP-CuS-FA also shows obvious contrast effect in PA, so it can be in-vivo tracked. This study may promote the further development of direct methods for targeted and image-guided collaborative PDT-PTT cancers [168].
In August 2020, Xie et al proposed a combined strategy of PTT based on BP and immunotherapy based on anti-CD47 antibody (acd47) to enhance the therapeutic effect of cancer [169]. In most cancers, monitoring the host's immune escape and metastasis through immunosuppressive cells in the tumor microenvironment to determine tumor resistance to immune checkpoint blockade remains a major challenge for cancer immunotherapy. Acd47 is a CD47/SIRP α axis blocker, which can induce moderate phagocytic activity and has a low response rate to monotherapy, leads to the failure of clinical trials. This strategy can be used as tumor specific immunotherapy to improve tumor specific immunity. BP combined with acd47 antagonist can activate innate and adaptive immunity, and promote local and systemic anti-cancer immune response, thus providing synergistic enhancement in inhibiting tumor progression and metastasis. This approach provides a promising strategy for enhancing immunotherapy.
In August 2020, Huang et al designed a new multifunctional therapeutic nano-platform combining aggregation-induced emission (AIE) PSs (NH2-PEG-TTPy) and BP nanosheets. Through electrostatic interaction, the water-soluble positively charged AIE PS NH2-PEG-TTP is coated on the BP nanosheet. The developed nano-platform BP@PEG-TTPy was based on the AIE-PS-BP structure. This method not only improves the biocompatibility and physiological stability of BP nanosheets, but also gives therapeutic nanoplatforms for strong FL emission and PDT capabilities in the NIR region. In-vitro and in-vivo evaluations show that the multifunctional BP@PEG-TTPy performs work well in cancer treatment involving NIR FLI-PTI dual imaging guided PDT-PTT and phototherapy.
In November 2020, Li et al prepared a novel multifunctional photo-responsive nano phototherapeutic agent CY@HBPN (hyaluronic acid is coated on the BPN loaded with NIR dye (cytophosphate)) for effective FL imaging guided phototherapy of triple negative breast cancer [170]. The results showed that hyaluronic acid modification can improve the stability and safety of BP nanoparticles, which also enables BPN to actively target CD44 overexpression tumor cells. In addition to being used as fluorescent contrast agent, when added to HBPN, the cavity like compounds also show enhanced photodynamic and photothermal efficiency, thus producing an effective anti-tumor effect. Especially in three-dimensional organs, CY@HBPN not only inhibited the growth of tumor ball, but also inhibited the recurrence of tumor after light damage. It should be noted that CY@HBPN preferentially accumulated in tumor tissues, thus effectively eradicating tumors in the mouse model under image-guided phototherapy. In general, CY@HBPN has high stability, safety, high photothermal conversion efficiency and anti-tumor effect. It is considered that CY@HBPN has been developed into an intelligent nano agent against breast cancer, anti-microbial infection and ophthalmic drugs.
4.2.2. Treatment of ND
In 2017, Liu et al found that BP nanosheets can be developed as neuroprotective nanomedicines for ND treatment: BP nanosheets are able to selectively capture Cu2+ among the common transition-metal ions (such as Ca2+, Mg2+, Fe2+, Fe3+, and Zn2+) in the human body. As Cu2+ can catalyze the formation of oxide species and enhance related pathological processes and endanger human health. BP nanosheets can also act as antioxidants to reduce the formation of cytotoxic ROS associated with Cu2+ instability under physiological conditions. In addition, in-vitro and in-vivo studies have shown that compared with traditional drug molecules, BP nanosheets can enhance the permeability of blood–brain barrier through photothermal effects, thereby overcome the shortcomings of unstable small chemicals or peptides, and improve the efficacy of drugs. In addition, the high stability and outstanding biocompatibility of BP nanosheets endow ND treatment with excellent biosafety. These exciting features make BP nanosheets a promising ND treatment option to regulate Cu2+ concentration and reduce symptoms related to oxidative stress. The results of this study can add new impetus to the biomedical application of BP [171].
5. Enhance the stability of BP
2D BP has great potential for application in biomedical field. However, the stability of phosphorus atoms on the surface or edge of BP is poor, and it is easy to degrade in air [172, 173]. Generally speaking, the lack of stability is one of the main factors restricting the application of BP. At present, there are four strategies to improve the stability of BP:
5.1. Encapsulation
Encapsulation is a method of adding an inert material on the surface of the BP as a protective layer to improve its stability. There are many kinds of inert materials reported previously.
In 2014, Na et al studied the reduction of current fluctuations in several layers of BP field effect transistors caused by Al2O3 passivation. In order to verify the influence of Al2O3 passivation on the device characteristics, the thermally annealed devices were measured and analyzed before and after passivation. The Raman spectra confirmed that when the BP flakes are passivated by Al2O3, and the passivated BP flakes could remain stable for over two months in ambient air [174]. In 2015, Kim et al used a double-layer cap with dielectric and fluoropolymer film to provide BP field effect transistors with strong air stability for several months [175]. In 2016, Pei et al used oxygen plasma etching to dilute the thick stripped phosphors layer by layer, and then passivated them with Al2O3 coating, which opened up a new way for the production of air-stable and high-quality monolayered or few-layered phosphorene samples [176]. In order to study the humidity sensing behavior of PET composed of multilayer BP, Miao Jinshui et al used 6 nm thick Al2O3 dielectric layer to passivate and stabilize the BP sensor. Comprehensive characteristics show that Al2O3 packaging can effectively inhibit BP degradation, thereby greatly improving the long-term stability of the device in ambient air [177]. Sinha et al developed a simple and general method for passivation of thin BP: firstly, large area high-quality single-layer hBN thin plate was grown by CVD, and then the mechanically peeled BP sheet prepared on Si substrate was covered with a single hBN sheet to protect the atomic layer BP sheet from degradation [178]. Graphene can also be used to passivate ultra-thin BP: in 2015, Doganov et al proved that atomically thin graphene and hBN can be used to passivate ultra-thin BP [179]. Later, Cai et al discovered that graphene and hBN can not only maintain the main electronic properties of BP by protecting them from structural and chemical degradation, but also can be used to adjust the carrier dynamics and optical properties of BP [180]. Ahmet Avsar et al constructed a fully packaged ultra-thin (to double-layer) BP field effect transistor manufactured under inert gas conditions by using graphene as source and drain electrodes and BN as an encapsulation layer. Excellent performance is realized in this field effect transistor (FET) with excellent stability of BP [181]. Zhao et al proposed a new effective passivation method: self-assembly of organic monolayers on BP by weak VDW epitaxy, so as to encapsulate BP precisely. The simulation results show that per 3, 4, 9, 10-tetracarboxylic dianhydride (PTCDA) does not affect the original electronic properties of BP, and can self-assemble on BP by VDW epitaxy to form a stable and close packed herringbone hydrogen bond network at about 400 K. At the same time, when the thickness of the PTCDA single layer is about 2 nm, BP can be well isolated from the surrounding O2 and H2O. Due to the high stability of the single layer PTCDA and the strong ability to resist the intrusion of the surrounding air, it is expected to be widely used as a passivation layer for other materials for improvement of stability [182]. In 2015, Bensong Wan et al fabricated p-type FETs with several layers BP flakes passivated by SiO2, in which the BP flakes are mechanically peeled from the bulk BP crystals. By passivating the BP with a SiO2 layer derived from inductively coupled plasma (ICP)-CVD, even after 1 week of BP treatment, the BP device can still generate a high on/off current ratio of over 600 and a high mobility of about 500 cm2 V−1 s−1 [183].
5.2. Functionalization
Chemical functionalization refers to the method to stabilize the BP structure by forming specific bonds between BP and related chemicals.
The earliest work on the formation of BP functional groups was reported by Ryder et al. They immersed BP nanosheets in an aryl diazonium salt solution, and formed a covalent bond with the BP through the aryl diazonium salt. The method stabilizes BP, and the results show that after three weeks of environmental exposure, the functionalization of covalent aryldiazonium can still inhibit the chemical degradation of BP [184]. In 2015, Zhao, et al synthesized titanium sulfonate ligands for surface coordination of BP. In contrast to the severe degradation observed by bare BP, the composite BP is relatively stable in both water dispersion and long-term exposure to air, which significantly prolongs the life of BP and promotes its practical application [185]. In 2016, Kumar et al synthesized BP nanosheets with poly-L-lysine to promote binding to the anti-Mb DNA aptamer generated on the nanostructured electrode. This strategy opens up a new way for bedside technology to allow multiple diagnosis of cardiovascular disease in complex human samples [186]. In 2016, Abellan et al proposed the concept of overall non-covalent organic functionalization of BP. Use electron-poor and polarizable polycyclic aromatic compounds (i.e. 7, 7, 8, 8-tetracyano-p-quinodimethane molecules and tailor-made per diimides) for BP. Wet chemical treatment can form a stable hybrid material covering and shielding the surface of several thin BP sheets with organic components [187]. In 2017, Guo et al used metal ion modification method to achieve the purpose of simultaneously enhancing the stability of BP chip and transistor performance. Ag+ can be adsorbed on the surface of BP by cation–π interaction, which passivates the lone pair P electrons, makes BP more stable in air. Therefore, the hole mobility of Ag+ modified BP FET increased from 796 to 1666 cm2 V s−1, and the on/off ratio was from 5.9 × 104 to –2.6 × 106 [188]. Maria Caporali et al used Ni to perform a new surface functionalization of the exfoliated BP, and the results showed that the nanoparticles were uniformly dispersed on the surface of the BP flakes: under environmental conditions, the stability to degradation of BP is improved [189]. In 2020, Lloret et al discovered that customized perylene diimides (PDI), which has an amido aromatic side chain, provide non-covalent functionalization for BP, the results showed that the introduction of aromatic groups leads to higher adsorption energy, which is mainly controlled by VDW interaction, which leads to closer packing of molecules on the surface of BP, further improve the stability of BP [190].
5.3. Liquid phase environmental protection
Liquid phase environment stabilization of BP is also one of the important technologies for stabilizing BP nanosheets [191–193].
Brent et al added the surfactant polyethylene glycol octyl anisole in water with BP dissolved. The head gene of polyethylene glycol octyl anisole can form a protective layer on the surface of BP to prevent the degradation of BP nanosheets. This method effectively enhances the stability of BP [194]. Wancheng Zhao et al exfoliated BP nanosheets in ionic liquid, and the prepared samples can be stable for one month under atmospheric environment. The study by Gonzalo Abellan et al showed that because ionic liquids can physically bond with the surface of BP, thereby improving the stability of BP, the usage of ionic liquids can stabilize the few-layer BP nanosheets for several months (<10 nm) [195].
5.4. Doping
Yang et al found that doping tellurium in BP can improve its stability. After three weeks of exposure to tellurium-doped BP, no significant degradation was observed [196]. In May 2018, Zhang, et al prepared tmsc1(trimethylsilyl) @ BP by modifying BP nanosheets with silicone tmsc1. The mechanism of the stability is that the relative positively charged tmsc1 can be coupled with the negatively charged BP, and the hydrophilic methylsilyl on the surface can protect the BP nanosheets from oxidation and moisture. Therefore, when modified by tmcsc1, BP showed higher zeta potential, more suitable size and better biomedical application dispersion [197]. In 2018, Xuan et al found that both nitrogen doping and hole carrier doping can improve the energy degeneracy of two-dimensional BP. Chemically inert substrates, such as graphene and hBN, can act synergistically with carrier doping to make BP more stable than other 2D allotropes, while frequently used metal substrates will severely reduce 2D BP stability [198] In 2019, Chang's et al found that sulfur (S)-doped BP nanosheets prepared by high-pressure synthesis followed by liquid stripping have better OER electrocatalytic activity and stability [199]. Recently, Xing et al proposed an in-situ encapsulated by thin-layer h-BN and surface modified by HfO2 or MgO provide an effective surface charge transfer doping and passivation scheme for several layers of BP. Both HfO2 and MgO can provide electronic doping effects on BP. The thin layer of h-BN can not only isolate BP from the surrounding environment, but also protect it from the destruction of metal oxide deposition [200].
6. Summary and outlook
In summary, this review summarizes the preparation methods for two-dimensional BP: top-down (such as mechanical cracking and liquid phase stripping) and bottom-up (such as CVD and wet chemical methods), and then introduces many interesting properties of BP such as biocompatibility, biodegradability, anisotropy, moderate carrier mobility and so on. The application of BP in biomedicine are introduced including medical diagnosis and medical treatment.
With the continuous efforts of scientists in recent years, the application of BP has become an attractive option for medical diagnosis and medical treatment. However, compared with other 2D materials, there is a long way to go for 2D BP. The first problem is that it is lack of stability. Therefore, it is necessary to find an effective method to effectively produce non-degradable and highly stable BP nanosheets. Another practical obstacle for industrial application of BP is that there is still no practical method for synthetization of large size BP flakes. Therefore, whether this problem could be perfectly settled is also crucial to the future development of BP.
Another obstacle for BP's clinical application is that the research on its degradation mechanism in human body is not enough. As we known, the main component of BP material is phosphorus, a rich element in the human body, therefore, BP can be degraded into phosphoric acid in-vivo, which is harmless to human body. However, for the clinical application of 2D BP, whether long-term usage could cause excessive phosphate ion poisoning (such as the loss of metal ions as calcium and magnesium) still needs to be tested and verified. The effect requires degradation, which is also contradictory to improving its stability. Therefore, it is also necessary to find a balance between stability and degradation performance.
The research of 2D BP is in its initial stage. As long as the scientific community continues for in-depth research, it is believed that these problems will be solved gradually, and 2D BP may be successfully applied in the field of biomedicine, and eventually enter the stage of clinical use. In the future, the application of BP in the field of cancer treatment can further combine gene therapy, immunotherapy and light-mediated anti-tumor methods such as PTT and photodynamic therapy to develop more diversified multi-modal cancer therapies. Combining laser ultrafast detection technology to develop an integrated platform for diagnosis and treatment, and explore new models and programs for cancer diagnosis and treatment.
Acknowledgments
The authors acknowledge the financial support from Natural Science Foundation of Shenzhen Science and technology innovation Commission (Grant Nos. JCYJ20180301170948918 and JCYJ 20190813102005655).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files). Data will be available from 28 February 2022.














