Abstract
Nuclear energy is a low-carbon, safe, efficient, and sustainable clean energy. The new generation of nuclear energy systems operate in harsher environments under higher working temperatures and irradiation doses, while traditional nuclear power materials cannot meet the requirements. The development of high-performance nuclear power materials is a key factor for promoting the development of nuclear energy. Oxide dispersion strengthened (ODS) steel contains a high number density of dispersed nano-oxides and defect sinks and exhibits excellent high temperature creep performance and irradiation swelling resistance. Therefore, ODS steel has been considered as one of the most promising candidate materials for fourth-generation nuclear fission reactor cladding tubes and nuclear fusion reactor blankets. The preparation process significantly influences microstructure of ODS steel. This paper reviews the development and perspective of several preparation processes of ODS steel, including the powder metallurgy process, improved powder metallurgy process, liquid metal forming process, hybrid process, and additive forging. This paper also summarizes and analyzes the relationship between microstructures and the preparation process. After comprehensive consideration, the powder metallurgy process is still the best preparation process for ODS steel. Combining the advantages and disadvantages of the above preparation processes, the trend applied additive forging for extreme manufacturing of large ODS steel components is discussed with the goal of providing a reference for the application and development of ODS steel in nuclear energy.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years, energy conservation and environmental protection have become common issues all over the world. As a result, clean and renewable energy has become more desirable. Nuclear energy is the only clean, low-carbon, safe, efficient, and sustainable baseload energy, which will play an important role in enhancing energy supply and security and solving environmental pollution problems [1–5]. The fourth-generation nuclear energy system operates at high temperature and pressure, high creep, strong corrosion, and high dose neutron irradiation. Under these conditions, the mechanical properties and irradiation resistance of traditional reactor structural materials can no longer meet the service requirements [6–8]. In order to make new generation nuclear power systems with the characteristics of sustainability, safety and reliability, economy, antiproliferation, and physical protection, nuclear power materials must have irradiation embrittlement and swelling resistance, strong corrosion resistance, good mechanical properties, and chemical compatibility with coolant at high temperatures [7, 9, 10]. Therefore, nuclear power material is one of the key factors restricting the development of nuclear energy and has become a research hotspot in this field.
The typical microstructures of oxide dispersion strengthened (ODS) steel include: sub-micron grain size, defect sink strengths near or greater than 1016 m−2, nano-oxides with average diameters <d> ≈ 1–5 nm, number density N≈ 1023−24 m−3 and volume fraction f ≈ 0.5%–1%, coherent and semi-coherent interface relationships, and misfit dislocations between the nano-oxides and matrix [11–16]. The dispersed nano-oxides in ODS steel hinders the movement of dislocations and grain boundaries, and the high temperature strength and service temperature increases significantly [17]. Plenty of phase interfaces between nano-oxides and matrix can effectively trap point defects and bubbles caused by the agglomeration of helium—which is a major product from nuclear reactions between the incident neutrons and the material—which enhances the recombination of point defects, suppresses the growth of bubbles, and greatly improves irradiation swelling resistance [14, 18]. Therefore, ODS steel has been considered as one of the most promising materials for fourth-generation nuclear fission reactor cladding tubes and nuclear fusion reactor blankets [19–22].
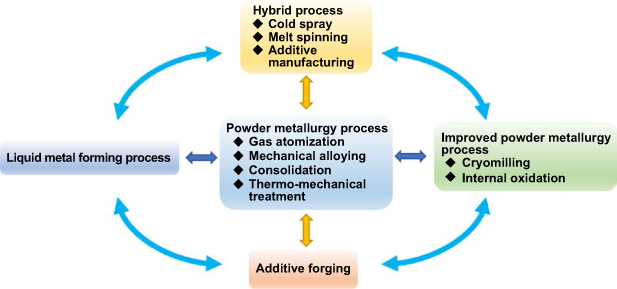
The excellent properties of ODS steel come from its unique microstructure, especially nano-oxides. However, the alloy composition and preparation process influence the formation of dispersed nano-oxides [23, 24]. Numerous studies regarding the formation mechanism of dispersed nano-oxides in ODS steel show that the preparation process significantly affects the average size, number density, and distribution of nano-oxides. The preparation process also determines the high temperature service performance and irradiation swelling resistance. This paper systematically summarizes the research progress on ODS steel preparation, including the powder metallurgy process, improved powder metallurgy process, liquid metal forming process, hybrid process, and additive forging, as shown in figure 1. Furthermore, the relationships between microstructures, properties, and the ODS steel preparation process are discussed. Finally, this paper proposes a preparation process that is expected to meet the requirements of high performance and homogenization of large-size ODS steel for nuclear power by combining the advantages and disadvantages of above preparation processes.
Figure 1. Classification of preparation processes toward ODS steel.
Download figure:
Standard image High-resolution image2. Powder metallurgy process
At present, the main preparation method for ODS steel is the powder metallurgy process, including gas atomization, mechanical alloying, canning, consolidation forming (e.g. hot isostatic pressing (HIP), spark plasma sintering, and hot extrusion), and thermo-mechanical treatment, as shown in figure 2. Mechanical alloying was first proposed by Benjamin of the International Nickel Corporation in the 1960s [25] and was widely used in various research fields, such as dispersion strengthened alloy materials, nonequilibrium materials, and nano-materials. Mechanical alloying is a process in which metal or alloy powders are repeatedly flattened, cold welded, fractured, and rewelded with a milling medium in a milling container for long periods [26], as shown in figure 3. During mechanical alloying, extremely stable oxides continuously dissolve into the matrix, increasing the solid solubility of alloying elements, which ultimately yields a supersaturated solid solution. Mechanical alloying is a severe deformation process that supplies more than the estimated 24 eV of energy needed to fully dissolve Y and O from a Y2O3 reference state [27]. Consolidation at high temperature generates a dense material and induces stable nano-oxides. Usually, higher consolidation temperatures are preferred to increase the bonding energy between powder particles and form more thermodynamically stable nano-oxides. Nano-oxide precipitation occurs rapidly at relevant consolidation temperatures above 800 °C [28]. However, with the increase of consolidation temperature, the size of nano-oxides increases and the volume fraction and the particle density decrease. The precipitates in ODS Fe-14Cr-1W steel occurred through structural and/or chemical evolution around 900 °C by in-situ small angle x-ray scattering during continuous heating up to 1100 °C [29]. The presence of Ti strongly affects particle chemistry and reduces the average particle diameter. The addition of Cr reduces the particles' surface energy, which lowers the rate of coarsening [30]. Therefore, despite the higher consolidation temperature (about 1150 °C), the alloy containing Cr and Ti still contains smaller oxide particles. Mechanical alloying is a complex process and some of the important process parameters affect the final constitution of the powder in terms of milling speed, ball-to-powder weight ratio, milling time, milling atmosphere, milling temperature, etc. In addition, mechanical alloying has the disadvantages of low efficiency, poor stability between batches, and susceptibility to grinding medium, container, and air pollution [31–33]. In order to minimize the disadvantages of mechanical alloying, scholars have proposed improved powder metallurgy processes called cryomilling and internal oxidation.
Figure 2. Schematic representation of ODS steel processing and fabrication paths by powder metallurgy process. Reprinted from [28], Copyright (2019), with permission from Elsevier.
Download figure:
Standard image High-resolution imageFigure 3. Interaction between ball media and alloy powder. Reprinted from [26], Copyright (2001), with permission from Elsevier.
Download figure:
Standard image High-resolution image2.1. Cryomilling
Mechanical alloying increases the temperature of alloy powders at room temperature, which can produce excessive welded particles and agglomerations, hindering atomic diffusion and reducing the number density of nano-oxides in ODS steel [34]. However, the brittleness of alloy powders improves at low temperatures. The cryomilling process takes advantage of cryogenic temperatures and conventional mechanical alloying. During this process, the alloy powders are milled in an attrition ball mill vessel under liquid nitrogen at cryogenic temperature [35]. There are several advantages to cryomilling compared to conventional mechanical alloying [34–39]. Cyromilling suppresses agglomeration and welding of alloy powders to the milling media. It also balances the crushing and cold welding of alloy powders, resulting in a more efficient milling outcome. The oxides can be completely dispersed through physical mechanisms. Cryomilling significantly reduces the milling time required to attain a supersaturated solid. The low temperature suppresses the annihilation of dislocations, making the accumulation of a higher dislocation density possible. This process minimizes oxidation reactions and inhibits recovery and recrystallization during milling, resulting in smaller grain sizes and increased nanostructure formation kinetics. The transition electron microscopy (TEM) images showing the nano-oxide particles of 14Cr-ODS steel produced by conventional mechanical alloying and −150 °C cryomilling are shown in figure 4. Cryomilling effectively changes the microstructure, and it produces homogeneous microstructures with narrow oxide particle grain boundary spacing and sub-micron-sized grains. The resulting microstructures exhibit a lower creep rate and higher creep strength [41]. The 14Cr-ODS steel produced by cryomilling exhibits superior oxidation resistance compared to that of conventional mechanical alloying. This difference is due to the fine grain size, small nano-oxides, and naturally formed oxide-film [40]. The mean diameter of nano-oxides below 10 nm decreases, and the number density of the particles increases as the mechanical alloying temperature decreases, according to small-angle neutron scattering analyses. The high temperature strength increases considerably as the mechanical alloying temperature decreases [42]. However, mechanical alloying at −150 °C significantly deteriorates the fracture toughness, which could be due to increased pore volume fraction and segregation at the grain boundaries [42].
Figure 4. TEM images of 14Cr-ODS steel produced by (a) conventional mechanical alloying and (b) −150 °C cryomilling. Reprinted from [40], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution image2.2. Internal oxidation
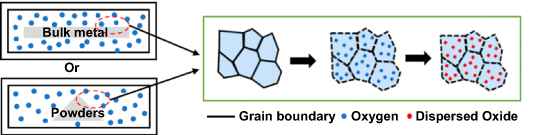
Internal oxidation is a process in which alloy powders or bulk metals are subjected to high temperature heat treatment under an oxygen atmosphere, so oxygen can diffuse into the matrix and combine with alloy elements to form oxides, thus avoiding the time intensive mechanical alloying step, as shown in figure 5. Schneibel et al [43] studied nano-scale oxide dispersoids by internal oxidation of Fe–Ti–Y intermetallics. Their results showed that in addition to Y2O3 and Y2Ti2O7 nano-oxides, large YFeO3 and Fe2TiO4 oxide particles were also produced by internal oxidation of Fe17Y2 and Fe11YTi intermetallics in a closed heat treatment environment where the Fe/Fe2O3 mixture provided oxygen partial pressure. In an environment where VO/V2O3 mixed powder served as the oxygen source, no oxide layer appeared on the microstructure surface of cast Fe-16Cr-0.2Al-0.05Y alloy after heat treatment at 1450 °C for 20 h, but there was a 2000 μm deep internal oxidation zone, and nano-scale Y–Al–O particles were present in the matrix [44].
Figure 5. Schematic representation of ODS steel produced by internal oxidation.
Download figure:
Standard image High-resolution imageThe diffusion distance of oxygen in a matrix is limited by the oxygen partial pressure, thus the number density and the homogeneity of nano-oxides in ODS steel prepared by internal oxidation is significantly lower than mechanical alloying. In order for oxygen to diffuse fully into matrix to form the desired microstructure, some scholars proposed improved internal oxidation methods:
(a) Gas atomization reaction synthesis (GARS) [45]: GARS is a high-flux rapid solidification process that utilizes a reactive atomization gas (Ar-O2) to surface oxidize nascent alloy droplets in-situ during the primary disintegration of molten metal in gas atomization. Rapid reaction kinetics promote the formation of a metastable surface oxide layer, which simultaneously provides a source of oxygen and a means of transporting large quantities of oxygen into a consolidated microstructure along prior particle boundaries and grain boundaries. Then, consolidation forming and high temperature heat treatment drive oxygen to combine with alloy elements to form highly stable and dispersed nano-oxides throughout the matrix. However, due to the rapid melting speed of the metallic matrix in the gas atomization process, the metallic matrix cannot fully react with oxygen. The metastable surface oxide layer that forms on the gas atomization powder has an heterogeneous distribution and thin thickness, resulting in ODS steel containing a lower number density of irregularly distributed nano-oxides.
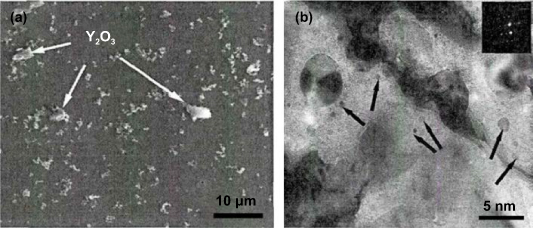
(b) Surface treatment of gas atomized powder followed by reactive synthesis (STARS): Gil and Ordás [32, 46] proposed STARS in response to the disadvantages of GARS. This method can improve the insufficient oxidation reaction in GARS by oxidizing gas atomization powders for a long time and ensuring the formation of a homogeneous oxide layer on the surface of alloy powders. The main steps include: 1. obtaining Ti and Y alloy powders by gas atomization; 2. formation of a homogenous metastable oxide layer of Cr2O3 and Fe2O3 on the surface of the gas atomization powders by oxidation reactions; 3. obtain oxygen by dissolving the metastable oxide layer so Cr2O3 and Fe2O3 diffuses into the matrix to form Y–Ti–O nano-oxides during consolidation via HIP; 4. residual oxides at a prior particle boundary are further decomposed by plastic deformation and heat treatment. The obtained nano-oxides have poor homogeneity and larger than average size, as shown in figure 6(a). The oxides do not completely decompose at low temperature HIP. Hence, a higher temperature heat treatment is required to fully decompose the residual oxides. However, the harmful effects of high temperature heat treatment, such as grain growth, heat-induced pore and aggregation of residual Ti (C, N), should be avoided.
Figure 6. TEM images of ODS steel prepared by (a) STARS and (b) internal oxidation to improve oxygen diffusion ability. Figure 6(a) reprinted from [46], Copyright (2018), with permission from Elsevier. Figure 6(b) reprinted from [47], Copyright (2019), with permission from Elsevier.
Download figure:
Standard image High-resolution image(c) Enhance oxygen diffusion capacity [48]: the diffusion of oxygen into matrix determines the homogeneity and number density of nano-oxides in ODS steel. The oxidated alloy powders increase dislocations and vacancy defects through short-term milling, which promotes oxygen diffusion. The resulting ODS steel contains a higher dislocation density, finer grain size, and more dispersed nano-oxide particles. The mechanical properties of the ODS steel obtained by above process were equivalent to those obtained through mechanical alloying, but the average size of the nano-oxides are above 20 nm [47], as shown in figure 6(b). The smaller the size of nano-oxides, the stronger the irradiation swelling resistance [14]. Hence, ODS steel prepared by this method has poor irradiation swelling resistance.
In addition, improved powder metallurgy processes also include two-step ball milling to promote the formation of nano-oxides [23] and the flake powder metallurgy process [49] and water atomization for cost reduction [50]. Although, improved powder metallurgy processes can minimize the disadvantages of mechanical alloying, the traditional powder metallurgy process is still a more suitable preparation process for ODS steel considering its comprehensive performance and preparation cost.
3. Liquid metal forming process
The liquid metal forming process is a process in which fine oxide powders are added to liquid steel and dispersed oxide particles are formed in the matrix [51]. The liquid metal forming process has attracted sustained attention attributed to its advantages, such as short process flow, low preparation cost, and large-scale preparation in a single batch. However, the agglomeration and coarsening of oxide particles are inevitable in melting and solidification [52]. The reasons for the above phenomenon are as follows [51, 53]: (a) the wettability of oxide particles is limited in liquid steel; (b) the density of oxides is generally lower than liquid steel and oxides are easy to suspend and form residue during smelting; and (c) the added oxides are thermally stable and are difficult to dissolve in smelting and solidification. In addition, the liquid metal forming process also needs to solve some complicated problems [53], such as how to disperse the added oxide particles in liquid steel, how to generate nano-oxides in a matrix, and how to prevent the generated oxides from dissolving or overreacting with the liquid alloy.
T91 steel and 9Cr low-activated steel strengthened by Y2O3 were prepared by directly adding micron Y2O3 particles into liquid steel. Y2O3 particles can be effectively added into a matrix according the results of tensile fracture and extraction. However, oxide particles agglomerated and coarsened, so the size was still on the micron-scale [54, 55], as shown in figure 7(a). Therefore, adding smaller oxide particles to the liquid steel forms smaller dispersed oxide particles in liquid steel. The wettability of oxide particles is a critical problem, Shi [55] proposed the intermediate alloy process combining the advantages of metallurgy and casting to improve wettability and bonding force with liquid steel. The intermediate alloy was prepared by sintering the mechanical alloy powder of Fe and Y2O3 particles. The T91 steel with dispersed nano-scale Y2O3 particles was realized by vacuum induction melting, as shown in figure 7(b). Verhiest et al [56] tried to use low-concentration and low-viscosity colloidal Y2O3 powder suspension as an additive to reduce agglomeration of Y2O3 in liquid steel. The results showed that simply adding colloidal Y2O3 with low-concentration and low-viscosity into liquid steel cannot uniformly disperse in the matrix. However, the addition of Y on the above basis improved the wettability of the Y2O3 particles in the liquid Fe–Cr alloy, and inhibited agglomeration, and promoted dispersive distribution of Y2O3.
Figure 7. (a) SEM image of extraction oxide of the 9Cr ODS steel produced by the direct casting method, (b) TEM image of the T91 ODS steel produced by intermediate alloy casting method [55]. No permission required.
Download figure:
Standard image High-resolution imageIn the powder metallurgy process, Y2O3 powders produce a kind of heterogeneous size distribution of particles and large flakes mixed with fine particles phenomenon in the ball mill. The morphology of Fe2O3 powders was controllable during ball milling, so it was used as an additive for mechanical alloying [57, 58]. Similarly, in the liquid metal forming process, oxides with thermal stability or melting points lower than Y2O3 can be added into the liquid steel containing Y as oxygen carriers. Meanwhile, the wettability between oxides and liquid steel can be improved. Hong et al [59] prepared 9Cr-ODS steel with Fe2O3 as the oxygen carrier. The main process flows are shown in figure 8: (a) Y powders were added to liquid steel and dissolved; (b) the oxygen carrier Fe2O3 powders were placed at the bottom of the mold; and (c) the liquid steel with Y was poured into the mold, and the oxygen was provided by dissolving Fe2O3 powder reacted with Y in the liquid steel to form uniformly distributed Y2O3. The above Fe2O3 powder dissolution and Y2O3 generation were completed simultaneously in a very short time during pouring and solidification, which inhibited the coarsening and agglomeration of Y2O3. This process avoids the mechanical alloying process, but the resulting oxides are of sub-micron scale, as shown in figure 9. Yan et al [60] proposed a prelaying powder casting technology based on the oxygen carrier casting technology and the preparation principle of rare earth steel. The liquid pure iron was alloyed by adding alloying elements at a certain oxygen concentration and then casted into the die containing Y, which promoted the reaction of Y and oxygen to form nano-oxides with the size of 1 ∼ 5 nm.
Figure 8. Schematic representation of producing ODS steel by the Fe2O3 oxygen carrier casting method. Reprinted from [59], Copyright (2019), with permission from Elsevier.
Download figure:
Standard image High-resolution imageFigure 9. (a) SEM of the cast microstructure of the 9Cr ODS steel produced by the oxygen carrier casting method; (b) high magnification SEM micrograph of the selected region in (a).
Download figure:
Standard image High-resolution imageWhen oxide particles mix with liquid steel, the oxide particles distribute heterogeneously due to the suspension caused by the density difference, which causes stress at the interface between the oxide particles and the matrix [61]. Oxide particles can be uniformly distributed in the liquid steel by auxiliary stirring during casting, but doing so causes agglomeration, hence noncontact stirring is required. Grants et al [62] used a magnetically driven tornado-like vortex formed by the superposition of two different alternating magnetic fields to agitate suspended particles to disperse them in liquid metal. Tang et al [61] used electromagnetic stirring to disperse pure Ti wires in liquid steel at multiple points and generated in-situ nano-oxides through internal oxidation, as shown in figure 10. The interfacial energy between the matrix and oxide particles decreased and the stability of the oxide particles improved due to the coherent or semi-coherent relationship between the oxide particles and the matrix.
Figure 10. Schematic of the processing method for forming nanoparticles in melt through electromagnetic stirring. Reprinted from [61], Copyright (2014), with permission from Elsevier.
Download figure:
Standard image High-resolution imageThe liquid metal forming process is a feasible ODS steel preparation process. Blending fine oxide particles with liquid steel and dispersing them in a matrix are still great challenges. However, the simplicity, low cost, and size scalability of the liquid metal forming process will continuously to be favored among researchers and encourage the development of the new liquid metal forming process.
4. Hybrid process
4.1. Cold spray
The cold spray process operates by propelling powder particles onto a substrate surface at supersonic velocities to form a near-net shape component or coating [63–65]. Bonding of particles occurs by plastic deformation and the adiabatic shear mechanism. Due to the lower particle deposition temperature, the original phase and compositional purity of the powders are preserved during deposition, and a high-density, nonoxidized coating or deposition layer can be produced. Benjamin et al [64] used the cold spray process to deposit gas atomized 14YWT powders on a rotating 6061-T6 aluminum tube mandrel, and obtained a cladding tube with a thickness of 1 mm by removing the 6061-T6 aluminum tube mandrel in an alkaline solution, as shown in figure 11. The cold spray process prevents a large number of extrusion and annealing steps and reduces the production cost associated with ODS steel cladding tubes. However, the low densification of ODS steel tubes and the low number density of nano-oxides result in poor mechanical properties.
Figure 11. Schematic representation of cold spraying technology [64]. Reproduced from [64]. CC BY-NC-ND 4.0 with permission.
Download figure:
Standard image High-resolution image4.2. Melt spinning
In the melt spinning process, a molten alloy is pressurized and sprayed onto a spinning drum to attain a rapid cooling rate inside a vacuum chamber, and the products come out as continuous thin ribbons and are collected on the other side of the chamber [35]. Due to the rapid cooling rate, melt spinning has been used to obtain supersaturated solid solutions containing fine precipitates. To form fine dispersed oxides in ribbons, the environment of melt spinning needs to contain oxygen partial pressure, so oxygen can diffuse into the liquid ribbon to form fine dispersed oxides. Hong et al [66] conducted melt spinning of Fe-1Y-1Ti to produce a uniformly distributed solid solution of Y and Ti. A ODS steel with rich Y oxides of less than 20 nm was obtained by heat treatment at 700 °C under the environment of Fe2O3 as the oxygen source, as shown in figure 12. However, the heterogeneous size of the nano-oxides and the existence of a small portion of permanent holes in matrix reduced the performance.
Figure 12. (a) Backscattered electron micrograph with inset image of the as-spun ribbon, (b) high-magnification scanning transmission electron microscopy (STEM) micrograph with inset energy dispersive X-Ray spectroscopy (EDX) elemental maps from the red rectangle area [66]. Reproduced from [66]. CC BY 4.0. Copyright 2017, The Author(s).
Download figure:
Standard image High-resolution image4.3. Additive manufacturing
Selective laser melting is developed based on the rapid prototyping method [67]. The product shape is decomposed into several layers, and the geometric shapes of each layer are continuously scanned by the laser beam on the powder bed covering with metal powders. Finally, the metal components form after melting and consolidation of the metal powders. The basic principle is shown in figure 13(a). Walker et al [68] prepared PM2000-ODS alloys containing nano-oxides with sizes ranging from 50 to 60 nm by selective laser melting. Boegelein et al [69] studied the tensile properties of thin-walled PM2000-ODS alloy components with different thicknesses prepared by selective laser melting. Their results showed that the tensile strength at room temperature was comparable to the recrystallized PM2000-ODS alloy prepared by conventional methods after heat treatment at 1200 °C for 1 h. Elodie et al [70] prepared 14Cr-ODS steel by optimizing selective laser melting process parameters (e.g. laser power, scanning speed, etc) with up to 98% densification and dispersed Y–Ti-rich oxide particles.
Figure 13. Schematic of ODS steel produced by (a) selective laser melting and (b) laser deposition [71]. Figure 13(a) reprinted from [67], Copyright (2017), with permission from Elsevier.
Download figure:
Standard image High-resolution imageIn selective laser melting, the fluidity of powders is a complex process, and the flow behavior is multi-dimensional and changeable [72, 73]. The morphology of powders should be spherical to promote the flow of powders. Laser deposition has a high deposition rate and a relatively wide process window. The coaxial powder nozzle equipped with an ultrasonic vibration system can make full use of irregular powder particles. At the same time, Ar gas is directly delivered to the laser focusing area to prevent oxide formation during melting [71]. The process setup is shown in figure 13(b). ODS steel prepared by the laser deposition method contains Y–Ti–O-type nano-oxides with an average size of 45 nm [71], and the nano-oxides and grains are larger than those of ODS steel prepared by powder metallurgy, which results in lower microhardness. The Y2O3 and HfO2 powders with 14Cr ferritic steel gas atomized alloy powders were alloyed by mechanical milling, respectively; then a 14Cr-ODS steel was prepared by laser deposition [74]. The results showed that both types of ODS steel had nano-oxides, but their number density was much lower than that of ODS steel prepared by powder metallurgy.
Alloy powders undergo severe plastic deformation during mechanical alloying, and the morphology is generally irregular and spherical. The irregular powders can cause unstable injection of the powder nozzle system in laser deposition and nonuniform layer thicknesses in selective laser melting [75]. Carlos et al [76] proposed a new process for preparing ODS steel powders by laser beam and producing fragmented nanoparticles, as shown in figure 14. The dispersed nanoparticles were produced by pulsed laser irradiation of nanoparticle aggregates in water and adsorbed on a ferrite stainless steel powder carrier by a pH controlled electrostatic interaction. The results of high temperature compression of ODS steel prepared by laser deposition via the above alloy powders showed excellent mechanical properties, but it was not suitable for the preparation of large ODS components.
Figure 14. Schematic representation of the preparation of ODS steel powder by laser beam producing fragmented nanoparticles. Reprinted from [76], Copyright (2018), with permission from Elsevier. Reprinted from [75], Copyright (2018), with permission from Elsevier.
Download figure:
Standard image High-resolution imageODS steel prepared by a hybrid process has a low densification, and the average size and dispersion degree of the nano-oxides are not as good as those prepared by powder metallurgy. The dispersed nano-oxides in ODS steel hinder the dislocation movement and significantly increase strength, which elevates the dynamic recrystallization temperature and increases the manufacturing difficulty of products with complex shapes. Cold spray, additive manufacturing, and other hybrid processes can reduce the mechanical properties of the materials, but they are helpful for manufacturing products with complex shapes. In conclusion, ODS steel prepared by hybrid processes is not expected to obtain smaller size and more homogeneously dispersed nano-oxides, achieves a compromise between the performance of the final product and the applicability and cost of industrial production.
5. Additive forging
At present, the powder metallurgy process can produce generally a few kilograms to dozens of kilograms of ODS steel. The liquid metal forming process can prepare hundreds of kilograms of ODS steel under the condition of compromised performance. Cold spray, additive manufacturing, and other hybrid processes are applicable for the preparation of small and complex ODS steel components. ODS steel is not suitable for fusion welding since local melting will fracture the elongated grain structure and destroy the dispersed nano-oxides in the matrix [77], which enhances the manufacturing difficulty of large ODS steel components. However, it is particularly important to prepare large-size ODS steel for application in nuclear power, especially the first wall structure of nuclear fusion reactors. The Institute of Metal Research of the Chinese Academy of Sciences first put forward a novel technology to manufacture heavy, high-quality forgings by additive forging in the world. Homogenized, small-sized, high-quality slabs are used as the base elements. After surface treatment, stacking, vacuum electron beam welding, and lager plastic deformation—characterized by pressure-forging and multi-directional forging at high temperature—the homogenized heavy forgings can be obtained with fully healed interfaces, which breaks through the traditional concept that a heavy forging must be created using a large steel ingot and realizes the new manufacturing technology of 'by making greatly small' [78–81]. This technology has been applied to 110-ton hydroelectric alloy steel spindles and giant stainless steel support rings (15.6 m in diameter) for nuclear power [81].
Zhou et al [82] studied the interfacial microstructure evolution and bonding mechanisms of 14Cr-ODS steel produced by additive forging. A schematic representation of the additive forging process for ODS steel is shown in figure 15. Owing to the large number of dispersed nano-oxides in ODS steel, a large strain gradient energy storage was formed near the bonding interface, which provided a driving force for the strain-induced grain boundary migration and led to the discontinuous dynamic recrystallization phenomenon. As the amount of deformation increases, the migration rate of the interface grain boundary increases, which promotes the long-range migration of the grain boundary and ultimately achieves metallurgical bonding, as shown in figure 16. Therefore, additive forging provides a new idea for the preparation of large ODS steel components. However, due to the special microstructure of ODS steel and the large deformation at high temperature during additive forging, the size, morphology, and distribution of nano-oxides in the interface area still require further exploration.
Figure 15. Schematic representation of the additive forging process for ODS steel.
Download figure:
Standard image High-resolution imageFigure 16. Microstructure evolution of bonded interfaces in 14Cr-ODS steel during metal additive forging. Reprinted from [82], Copyright (2019), with permission from Elsevier. Reprinted from [83], Copyright (2019), with permission from Elsevier.
Download figure:
Standard image High-resolution image6. Conclusion and prospect
The excellent performance of ODS steel is derived from the highly dispersed nano-oxides or clusters in the matrix. However, the preparation process significantly affects the average size, number density, and dispersion degree of nano-oxides. It also determines the high-temperature creep performance and irradiation swelling resistance. Compared with the powder metallurgy process, cryomilling can reduce agglomeration of alloy powders and obtain homogeneous microstructures and smaller nano-oxides, but the cost of preparation is higher. Internal oxidation avoids the shortcomings of mechanical alloying, but due to the extent of internal oxidation and the limitation of oxygen partial pressure, the distribution of nano-oxides is nonuniform, the size is large, and the number density is lower. How to promote the diffusion capacity of oxygen and the homogeneity of nano-oxides will be the critical focus for obtaining special microstructures of ODS steel. The simplicity, low cost, and size scalability of the liquid metal forming process is a feasible method to prepare large-size ODS steel. However, blending fine oxide particles with liquid steel and dispersing them in the matrix still present significant challenges. ODS steel prepared by cold spraying, melt spinning, and additive manufacturing has lower densification. The size and dispersion degree of nano-oxides are not as good as those prepared by powder metallurgy. However, these method have the potential to prepare products with complex shapes, which is the compromise between the performance of the final product and the applicability and cost of industrial manufacturing. In addition, the material database of ODS steel obtained by the above preparation processes is incomplete, and the mechanical properties and microstructures should be further studied to enrich the database. After comprehensive consideration, powder metallurgy process is the best process to prepare ODS steel.
It is very important to prepare large-size ODS steel for promoting the application of ODS steel in nuclear power. At the same time, ensuring the formation and stability of nano-oxide particles and controlling their interfacial relationship with the matrix for large-size ODS steel require further study. Additive forging provides a new idea for the preparation of large ODS steel components. Although the joint interface of additive forging could achieve metallurgical bonding by dynamic recrystallization, due to the special microstructure of ODS steel and the large deformation at high temperature during the additive forging, the size, morphology, and distribution of nano-oxides in the interface area still require further exploration. Moreover, the mechanical properties of the interface should be evaluated to ensure that the properties of the interface are equivalent to the matrix, which will lay a foundation for the application of additive forging in ODS steel. With the development of ODS steel preparation processes, homogenized high-performance ODS steel slabs are obtained by the liquid metal forming process and then homogenized large-size ODS steel components are prepared by additive forging, which promotes the engineering application of ODS steel in nuclear power and other harsher service environment fields.
Acknowledgments
This work was supported by the National Key Research and Development Program [Grant No. 2018YFA0702900], the National Natural Science Foundation of China [Grant No. 51774265], the National Science and Technology Major Project of China [Grant No. 2019ZX06004010], the Strategic Priority Research Program of the Chinese Academy of Sciences [Grant No. XDC04000000], LingChuang Research Project of China National Nuclear Corporation, Program of CAS Interdisciplinary Innovation Team, and Youth Innovation Promotion Association, CAS.