Abstract
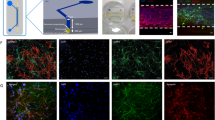
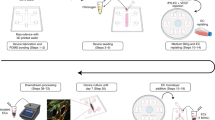
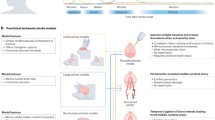
The therapeutic efficacy of stem cells transplanted into an ischaemic brain depends primarily on the responses of the neurovascular unit. Here, we report the development and applicability of a functional neurovascular unit on a microfluidic chip as a microphysiological model of ischaemic stroke that recapitulates the function of the blood–brain barrier as well as interactions between therapeutic stem cells and host cells (human brain microvascular endothelial cells, pericytes, astrocytes, microglia and neurons). We used the model to track the infiltration of a number of candidate stem cells and to characterize the expression levels of genes associated with post-stroke pathologies. We observed that each type of stem cell showed unique neurorestorative effects, primarily by supporting endogenous recovery rather than through direct cell replacement, and that the recovery of synaptic activities is correlated with the recovery of the structural and functional integrity of the neurovascular unit rather than with the regeneration of neurons.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding author on reasonable request.
References
Wang, Y. & Cai, Y. Obtaining human ischemic stroke gene expression biomarkers from animal models: a cross-species validation study. Sci. Rep. 6, 29693–29702 (2016).
Stonesifer, C. et al. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 158, 94–131 (2017).
Wechsler, L. R., Bates, D., Stroemer, P., Andrews-Zwilling, Y. S. & Aizman, I. Cell therapy for chronic stroke. Stroke 49, 1066–1074 (2018).
McGonigle, P. & Ruggeri, B. Animal models of human disease: challenges in enabling translation. Biochem. Pharmacol. 87, 162–171 (2014).
Savitz, S. I. et al. Stem cells as an emerging paradigm in stroke 3: enhancing the development of clinical trials. Stroke 45, 634–639 (2014).
Woodruff, T. M. et al. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 6, 11–29 (2011).
Del Zoppo, G. The neurovascular unit in the setting of stroke. J. Intern. Med. 267, 156–171 (2010).
Sivandzade, F. & Cucullo, L. In-vitro blood–brain barrier modeling: a review of modern and fast-advancing technologies. J. Cereb. Blood Flow. Metab. 38, 1667–1681 (2018).
Prabhakarpandian, B. et al. SyM-BBB: a microfluidic blood brain barrier model. Lab Chip 13, 1093–1101 (2013).
Herland, A. et al. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS ONE 11, e0150360 (2016).
Wevers, N. R. et al. A perfused human blood–brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 15, 23 (2018).
Grifno, G. N. et al. Tissue-engineered blood-brain barrier models via directed differentiation of human induced pluripotent stem cells. Sci. Rep. 9, 13957 (2019).
Ahn, S. I. et al. Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 11, 175 (2020).
Kim, S., Lee, H., Chung, M. & Jeon, N. L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13, 1489–1500 (2013).
Soofi, S. S., Last, J. A., Liliensiek, S. J., Nealey, P. F. & Murphy, C. J. The elastic modulus of Matrigel™ as determined by atomic force microscopy. J. Struct. Biol. 167, 216–219 (2009).
Budday, S. et al. Mechanical characterization of human brain tissue. Acta Biomater. 48, 319–340 (2017).
Uemura, M. et al. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell‐derived neural precursor cells. J. Neurosci. Res. 88, 542–551 (2010).
Yu, Z. et al. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis. 9, 945–956 (2018).
Nakagawa, S. et al. A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 54, 253–263 (2009).
Dejana, E. Endothelial cell–cell junctions: happy together. Nat. Rev. Mol. Cell Biol. 5, 261–270 (2004).
Lee, C. S. & Leong, K. W. Advances in microphysiological blood-brain barrier (BBB) models towards drug delivery. Curr. Opin. Biotechnol. 66, 78–87 (2020).
Mayhan, W. G. & Heistad, D. D. Permeability of blood-brain barrier to various sized molecules. Am. J. Physiol. 248, H712–H718 (1985).
Srinivasan, B. et al. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 20, 107–126 (2015).
Booth, R. & Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 12, 1784–1792 (2012).
Papademetriou, I., Vedula, E., Charest, J. & Porter, T. Effect of flow on targeting and penetration of angiopep-decorated nanoparticles in a microfluidic model blood-brain barrier. PLoS ONE 13, e0205158 (2018).
Walter, F. R. et al. A versatile lab-on-a-chip tool for modeling biological barriers. Sens. Actuators B 222, 1209–1219 (2016).
Brown, J. A. et al. Recreating blood-brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor. Biomicrofluidics 9, 054124 (2015).
Wang, Y. I., Abaci, H. E. & Shuler, M. L. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 114, 184–194 (2017).
Helms, H. C. et al. In vitro models of the blood–brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow. Metab. 36, 862–890 (2016).
Yeste, J. et al. Geometric correction factor for transepithelial electrical resistance measurements in transwell and microfluidic cell cultures. J. Phys. D Appl. Phys. 49, 375401 (2016).
Foo, L. C. et al. Development of a method for the purification and culture of rodent astrocytes. Neuron 71, 799–811 (2011).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Hakim, A. M. Ischemic penumbra: the therapeutic window. Neurology 51, S44–S46 (1998).
Heiss, W.-D. et al. Progressive derangement of periinfarct viable tissue in ischemic stroke. J. Cereb. Blood Flow. Metab. 12, 193–203 (1992).
Stankowski, J. N. & Gupta, R. Therapeutic targets for neuroprotection in acute ischemic stroke: lost in translation? Antioxid. Redox Signal. 14, 1841–1851 (2011).
Yang, L., Shah, K. K. & Abbruscato, T. J. An in vitro model of ischemic stroke. Methods Mol. Biol. 814, 451–466 (2012).
Shi, H. Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr. Med. Chem. 16, 4593–4600 (2009).
Mattson, M. P., Culmsee, C. & Yu, Z. F. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 301, 173–187 (2000).
Bereczki, J., Balla, J. & Bereczki, D. Heme oxygenase-1: clinical relevance in ischemic stroke. Curr. Pharm. Des. 24, 2229–2235 (2018).
Boshuizen, M. C. & Steinberg, G. K. Stem cell–based immunomodulation after stroke: effects on brain repair processes. Stroke 49, 1563–1570 (2018).
Jin, R., Yang, G. & Li, G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 87, 779–789 (2010).
Park, J. S., Bateman, M. C. & Goldberg, M. P. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol. Dis. 3, 215–227 (1996).
Schmued, L. C., Albertson, C. & Slikker, W. Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 751, 37–46 (1997).
Besse, A. et al. Personalized medicine approach confirms a milder case of ABAT deficiency. Mol. Brain 9, 93 (2016).
Okaty, B. W., Miller, M. N., Sugino, K., Hempel, C. M. & Nelson, S. B. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J. Neurosci. 29, 7040–7052 (2009).
Lai, T. W., Zhang, S. & Wang, Y. T. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog. Neurobiol. 115, 157–188 (2014).
Cameron, M. et al. Calcium imaging of AM dyes following prolonged incubation in acute neuronal tissue. PLoS ONE 11, e0155468 (2016).
Marambaud, P., Dreses-Werringloer, U. & Vingtdeux, V. Calcium signaling in neurodegeneration. Mol. Neurodegener. 4, 20 (2009).
Sneyd, J. et al. On the dynamical structure of calcium oscillations. Proc. Natl Acad. Sci. USA 114, 1456–1461 (2017).
Arundine, M. & Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34, 325–337 (2003).
Weksler, B. et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19, 1872–1874 (2005).
Tornavaca, O. et al. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J. Cell Biol. 208, 821–838 (2015).
Mathiu, O., van der Meer, A. D., JungáKim, H., van der Helm, M. W. & den Berg, A. Measuring direct current trans-epithelial electrical resistance in organ-on-a-chip microsystems. Lab Chip 15, 745–752 (2015).
Talwar, T. & Srivastava, M. V. P. Role of vascular endothelial growth factor and other growth factors in post-stroke recovery. Ann. Indian Acad. Neurol. 17, 1–6 (2014).
Sandoval, K. E. & Witt, K. A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 32, 200–219 (2008).
Carlos, T., Clark, R., Franicola-Higgins, D., Schiding, J. & Kochanek, P. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J. Leukoc. Biol. 61, 279–285 (1997).
DeStefano, J. G., Xu, Z. S., Williams, A. J., Yimam, N. & Searson, P. C. Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs). Fluids Barriers CNS 14, 20–34 (2017).
Colgan, O. C. et al. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am. J. Physiol. 292, 3190–3197 (2007).
Sweeney, M. D., Ayyadurai, S. & Zlokovic, B. V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19, 771–783 (2016).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol. Neurodegener. 5, 32 (2010).
Sá-Pereira, I., Brites, D. & Brito, M. A. Neurovascular unit: a focus on pericytes. Mol. Neurobiol. 45, 327–347 (2012).
Abbott, N. J., Rönnbäck, L. & Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006).
Papadopoulos, M. C. & Verkman, A. S. Aquaporin-4 and brain edema. Pediatr. Nephrol. 22, 778–784 (2007).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Kimelberg, H. K. & Nedergaard, M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7, 338–353 (2010).
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010).
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 (2017).
Guruswamy, R. & ElAli, A. Complex roles of microglial cells in ischemic stroke pathobiology: new insights and future directions. Int. J. Mol. Sci. 18, 496–511 (2017).
Taib, T. et al. Neuroinflammation, myelin and behavior: temporal patterns following mild traumatic brain injury in mice. PLoS ONE 12, e0184811 (2017).
Matt, S. M., Lawson, M. A. & Johnson, R. W. Aging and peripheral lipopolysaccharide can modulate epigenetic regulators and decrease IL-1β promoter DNA methylation in microglia. Neurobiol. Aging 47, 1–9 (2016).
Jalland, C. M. et al. Neil3 induced neurogenesis protects against prion disease during the clinical phase. Sci. Rep. 6, 37844–37852 (2016).
Patel, A. R., Ritzel, R., McCullough, L. D. & Liu, F. Microglia and ischemic stroke: a double-edged sword. Int. J. Physiol. Pathophysiol. Pharmacol. 5, 73–90 (2013).
Walker, D. G. & Lue, L.-F. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 7, 56–64 (2015).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Picascia, A., Grimaldi, V., Iannone, C., Soricelli, A. & Napoli, C. Innate and adaptive immune response in stroke: focus on epigenetic regulation. J. Neuroimmunol. 289, 111–120 (2015).
Junger, W. G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 11, 201–212 (2011).
Oliveira, A., Illes, P. & Ulrich, H. Purinergic receptors in embryonic and adult neurogenesis. Neuropharmacology 104, 272–281 (2016).
Marei, H. E. M. Potential of stem cell-based therapy for ischemic stroke. Front. Neurol. 9, 34–40 (2018).
Szklarczyk, D. et al. Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Naylor, A. J. et al. A differential role for CD248 (Endosialin) in PDGF-mediated skeletal muscle angiogenesis. PLoS ONE 9, e107146 (2014).
Sun, J. & Nan, G. The mitogen-activated protein kinase (MAPK) signaling pathway as a discovery target in stroke. J. Mol. Neurosci. 59, 90–98 (2016).
Kanehisa, M., Sato, Y., Furumichi, M., Morishima, K. & Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 47, D590–D595 (2019).
Pereda, A. E. Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 15, 250–263 (2014).
Janowski, M., Wagner, D.-C. & Boltze, J. Stem cell–based tissue replacement after stroke: factual necessity or notorious fiction? Stroke 46, 2354–2363 (2015).
Ohab, J. J. & Carmichael, S. T. Poststroke neurogenesis: emerging principles of migration and localization of immature neurons. Neuroscientist 14, 369–380 (2008).
Campisi, M. et al. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180, 117–129 (2018).
Brown, J. A. et al. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J. Neuroinflammation 13, 306 (2016).
Sances, S. et al. Human iPSC-derived endothelial cells and microengineered organ-chip enhance neuronal development. Stem Cell Rep. 10, 1222–1236 (2018).
Vatine, G. D. et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 24, 995–1005 (2019).
Xu, L., Nirwane, A. & Yao, Y. Basement membrane and blood–brain barrier. Stroke Vasc. Neurol. 4, 78–82 (2019).
Eddington, D. T., Puccinelli, J. P. & Beebe, D. J. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sens. Actuators B 114, 170–172 (2006).
Halldorsson, S., Lucumi, E., Gómez-Sjöberg, R. & Fleming, R. M. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 63, 218–231 (2015).
Ma, X. et al. Injection molding and characterization of PMMA-based microfluidic devices. Microsyst. Technol. 26, 1317–1324 (2020).
Daadi, M. M., Maag, A.-L. & Steinberg, G. K. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS ONE 3, e1644 (2008).
Offner, H., Vandenbark, A. & Hurn, P. D. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience 158, 1098–1111 (2009).
Ajami, N. E. et al. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc. Natl Acad. Sci. USA 114, 10990–10995 (2017).
Wang, C., Baker, B. M., Chen, C. S. & Schwartz, M. A. Endothelial cell sensing of flow direction. Arterioscler. Thromb. Vasc. Biol. 33, 2130–2136 (2013).
Wang, Y. I. & Shuler, M. L. UniChip enables long-term recirculating unidirectional perfusion with gravity-driven flow for microphysiological systems. Lab Chip 18, 2563–2574 (2018).
Rikhtegar, R. et al. Stem cell-based cell therapy for neuroprotection in stroke: a review. J. Cell. Biochem. 120, 8849–8862 (2019).
Huertas-Vazquez, A., Leon-Mimila, P. & Wang, J. Relevance of multi-omics studies in cardiovascular diseases. Front. Cardiovasc. Med. 6, 91 (2019).
Mi, S., Du, Z., Xu, Y. & Sun, W. The crossing and integration between microfluidic technology and 3D printing for organ-on-chips. J. Mater. Chem. B 6, 6191–6206 (2018).
von Bartheld, C. S., Bahney, J. & Herculano‐Houzel, S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J. Comp. Neurol. 524, 3865–3895 (2016).
Dore-Duffy, P. et al. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc. Res. 60, 55–69 (2000).
Luissint, A.-C., Artus, C., Glacial, F., Ganeshamoorthy, K. & Couraud, P.-O. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 9, 23 (2012).
Vormann, M. K. et al. Nephrotoxicity and kidney transport assessment on 3D perfused proximal tubules. AAPS J. 20, 90 (2018).
Curry, F., Huxley, V. & Adamson, R. Permeability of single capillaries to intermediate-sized colored solutes. Am. J. Physiol. 245, H495–H505 (1983).
Haase, K., Gillrie, M. R., Hajal, C. & Kamm, R. D. Pericytes contribute to dysfunction in a human 3D model of placental microvasculature through VEGF‐Ang‐Tie2 signaling. Adv. Sci. 6, 1900878 (2019).
Shin, Y. et al. Blood–brain barrier dysfunction in a 3D in vitro model of Alzheimer’s disease. Adv. Sci. 6, 1900962 (2019).
Lee, S. W. L. et al. Modeling nanocarrier transport across a 3D in vitro human blood‐brain–barrier microvasculature. Adv. Healthc. Mater. 9, e1901486 (2020).
Boussommier-Calleja, A. et al. The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model. Biomaterials 198, 180–193 (2019).
Rodríguez-Frutos, B. et al. Stem cell therapy and administration routes after stroke. Transl. Stroke Res. 7, 378–387 (2016).
Acknowledgements
We thank H. Suh for editing the manuscript; and T. Klein, J. Saunders and S. Saunders for their support. W.L. was supported by NIH National Center for Advancing Translational Science Clinical and Translational Science Award at the Stanford Child Health Research Institute (UL1 TR001085) and NIH National Cancer Institute career development award (K25CA201545).
Author information
Authors and Affiliations
Contributions
Z.L. and K.-M.K. designed and performed the experiments, and analysed and interpreted the data; J.P. designed experiment; H.-J.J. and H.W. performed the experiments; W.L. conceived and supervised the project, designed and performed experiments, and analysed and interpreted the data. The manuscript was mainly written by W.L. with contributions from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables and references.
Rights and permissions
About this article
Cite this article
Lyu, Z., Park, J., Kim, KM. et al. A neurovascular-unit-on-a-chip for the evaluation of the restorative potential of stem cell therapies for ischaemic stroke. Nat Biomed Eng 5, 847–863 (2021). https://doi.org/10.1038/s41551-021-00744-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00744-7
This article is cited by
-
Neuropathogenesis-on-chips for neurodegenerative diseases
Nature Communications (2024)
-
Vascularized organoid-on-a-chip: design, imaging, and analysis
Angiogenesis (2024)
-
Blood–brain barrier injury and neuroinflammation induced by SARS-CoV-2 in a lung–brain microphysiological system
Nature Biomedical Engineering (2023)
-
Neuroprotective Effects of Conditioned Medium of Mesenchymal Stem Cells (MSC-CM) as a Therapy for Ischemic Stroke Recovery: A Systematic Review
Neurochemical Research (2023)
-
Advances in brain barriers and brain fluids research in 2021: great progress in a time of adversity
Fluids and Barriers of the CNS (2022)