Abstract
Purpose
To validate the use of synthetic magnetic resonance imaging (SyMRI) volumetry by comparing with child-optimized SPM 12 volumetry in 3 T pediatric neuroimaging.
Methods
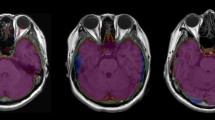
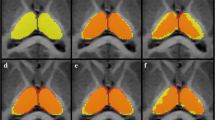
In total, 106 children aged 4.7–18.7 years who underwent both synthetic and 3D T1-weighted imaging and had no abnormal imaging/neurologic findings were included for the SyMRI vs. SPM T1-only segmentation (SPM T1). Forty of the 106 children who underwent an additional 3D T2-weighted imaging were included for the SyMRI vs. SPM multispectral segmentation (SPM multi). SPM segmentation using an age-appropriate atlas and inverse-transforming template-space intracranial mask was compared with SyMRI segmentation. Volume differences between SyMRI and SPM T1 were plotted against age to evaluate the influence of age on volume difference.
Results
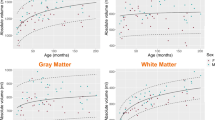
Measurements derived from SyMRI and two SPM methods showed excellent agreements and strong correlations except for the CSF volume (CSFV) (intraclass correlation coefficients = 0.87–0.98; r = 0.78–0.96; relative volume difference other than CSFV = 6.8–18.5% [SyMRI vs. SPM T1] and 11.3–22.7% [SyMRI vs. SPM multi]). Dice coefficients of all brain tissues (except CSF) were in the range 0.78–0.91. The Bland–Altman plot and age-related volume difference change suggested that the volume differences between the two methods were influenced by the volume of each brain tissue and subject’s age (p < 0.05).
Conclusion
SyMRI and SPM segmentation results were consistent except for CSFV, which supports routine clinical use of SyMRI-based volumetry in pediatric neuroimaging. However, caution should be taken in the interpretation of the CSF segmentation results.







Similar content being viewed by others
References
Fischl B (2012) FreeSurfer. Neuroimage 62:774–781
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62:782–790
Ashburner J (2012) SPM: a history. Neuroimage 62:791–800
West J, Warntjes JB, Lundberg P (2012) Novel whole brain segmentation and volume estimation using quantitative MRI. Eur Radiol 22:998–1007
Warntjes JB, Leinhard OD, West J, Lundberg P (2008) Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reson Med 60:320–329
Ji S, Yang D, Lee J, Choi SH, Kim H, Kang KM (2020) Synthetic MRI: technologies and applications in neuroradiology. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27440
Goncalves FG, Serai SD, Zuccoli G (2018) Synthetic brain MRI: review of current concepts and future directions. Top Magn Reson Imaging 27:387–393
West J, Blystad I, Engstrom M, Warntjes JB, Lundberg P (2013) Application of quantitative MRI for brain tissue segmentation at 1.5 T and 3.0 T field strengths. PLoS One 8:e74795
Vagberg M, Lindqvist T, Ambarki K et al (2013) Automated determination of brain parenchymal fraction in multiple sclerosis. AJNR Am J Neuroradiol 34:498–504
Hagiwara A, Hori M, Cohen-Adad J et al (2019) Linearity, bias, intrascanner repeatability, and interscanner reproducibility of quantitative multidynamic multiecho sequence for rapid simultaneous relaxometry at 3 T: a validation study with a standardized phantom and healthy controls. Invest Radiol 54:39–47
Granberg T, Uppman M, Hashim F et al (2016) Clinical feasibility of synthetic MRI in multiple sclerosis: a diagnostic and volumetric validation study. AJNR Am J Neuroradiol 37:1023–1029
Ambarki K, Lindqvist T, Wahlin A et al (2012) Evaluation of automatic measurement of the intracranial volume based on quantitative MR imaging. AJNR Am J Neuroradiol 33:1951–1956
McAllister A, Leach J, West H, Jones B, Zhang B, Serai S (2017) Quantitative synthetic MRI in children: normative intracranial tissue segmentation values during development. AJNR Am J Neuroradiol 38:2364–2372
Serai SD, Dudley J, Leach JL (2019) Comparison of whole brain segmentation and volume estimation in children and young adults using SPM and SyMRI. Clin Imaging 57:77–82
Kim HG, Moon WJ, Han J, Choi JW (2017) Quantification of myelin in children using multiparametric quantitative MRI: a pilot study. Neuroradiology 59:1043–1051
Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE (2012) Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp 33:1987–2002
Lee SM, Choi YH, You SK et al (2018) Age-related changes in tissue value properties in children: simultaneous quantification of relaxation times and proton density using synthetic magnetic resonance imaging. Invest Radiol 53:236–245
Andica C, Hagiwara A, Hori M et al (2019) Review of synthetic MRI in pediatric brains: basic principle of MR quantification, its features, clinical applications, and limitations. J Neuroradiol 46:268–275
Heinen R, Bouvy WH, Mendrik AM, Viergever MA, Biessels GJ, de Bresser J (2016) Robustness of automated methods for brain volume measurements across different MRI field strengths. PLoS One 11:e0165719
Hansen TI, Brezova V, Eikenes L, Haberg A, Vangberg TR (2015) How does the accuracy of intracranial volume measurements affect normalized brain volumes? Sample size estimates based on 966 subjects from the HUNT MRI cohort. AJNR Am J Neuroradiol 36:1450–1456
Keihaninejad S, Heckemann RA, Fagiolo G et al (2010) A robust method to estimate the intracranial volume across MRI field strengths (1.5T and 3T). Neuroimage 50:1427–1437
Malone IB, Leung KK, Clegg S et al (2015) Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage 104:366–372
Lindig T, Kotikalapudi R, Schweikardt D et al (2018) Evaluation of multimodal segmentation based on 3D T1-, T2- and FLAIR-weighted images - the difficulty of choosing. Neuroimage 170:210–221
Kotikalapudi R, Martin P, Marquetand J, Lindig T, Bender B, Focke NK (2018) Systematic assessment of multispectral voxel-based morphometry in previously MRI-negative focal epilepsy. AJNR Am J Neuroradiol 39:2014–2021
Evans AC, Brain Development Cooperative G (2006) The NIH MRI study of normal brain development. Neuroimage 30:184–202
Wilke M, Holland SK, Altaye M, Gaser C (2008) Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage 41:903–913
Terwee CB, Bot SD, de Boer MR et al (2007) Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 60:34–42
Biffen SC, Warton CMR, Dodge NC et al (2020) Validity of automated FreeSurfer segmentation compared to manual tracing in detecting prenatal alcohol exposure-related subcortical and corpus callosal alterations in 9- to 11-year-old children. Neuroimage Clin 28:102368
Guenette JP, Stern RA, Tripodis Y et al (2018) Automated versus manual segmentation of brain region volumes in former football players. Neuroimage Clin 18:888–896
Andica C, Hagiwara A, Hori M et al (2018) Automated brain tissue and myelin volumetry based on quantitative MR imaging with various in-plane resolutions. J Neuroradiol 45:164–168
Saccenti L, Andica C, Hagiwara A et al (2019) Brain tissue and myelin volumetric analysis in multiple sclerosis at 3T MRI with various in-plane resolutions using synthetic MRI. Neuroradiology 61:1219–1227
Zijdenbos AP, Dawant BM, Margolin RA, Palmer AC (1994) Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans Med Imaging 13:716–724
Nordenskjold R, Malmberg F, Larsson EM et al (2013) Intracranial volume estimated with commonly used methods could introduce bias in studies including brain volume measurements. Neuroimage 83:355–360
Phan TV, Smeets D, Talcott JB, Vandermosten M (2018) Processing of structural neuroimaging data in young children: bridging the gap between current practice and state-of-the-art methods. Dev Cogn Neurosci 33:206–223
Mills KL, Tamnes CK (2014) Methods and considerations for longitudinal structural brain imaging analysis across development. Dev Cogn Neurosci 9:172–190
Acknowledgements
We thank Sung Won Youn and Ho Kyun Kim of Catholic University of Daegu Medical School for technical assistance.
Funding
The study was supported by a grant from the National Research Foundation of Korea (NRF-2017R1C1B5075974).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
For this type of retrospective study, formal consent is not required.
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, S.M., Kim, E., You, S.K. et al. Clinical adaptation of synthetic MRI-based whole brain volume segmentation in children at 3 T: comparison with modified SPM segmentation methods. Neuroradiology 64, 381–392 (2022). https://doi.org/10.1007/s00234-021-02779-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02779-8