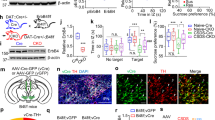
Abstract
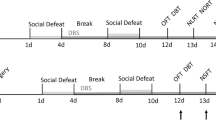
Numerous human clinical studies have suggested that decreased locomotor activity is a common symptom of major depressive disorder (MDD), as well as other psychiatric diseases. In MDD, the midbrain ventral tegmental area (VTA) dopamine (DA) neurons are closely related to regulate the information processing of reward, motivation, cognition, and aversion. However, the neural circuit mechanism that underlie the relationship between VTA-DA neurons and MDD-related motor impairments, especially hypolocomotion, is still largely unknown. Herein, we investigate how the VTA-DA neurons contribute to the hypolocomotion performance in chronic social defeat stress (CSDS), a mouse model of depression-relevant neurobehavioral states. The results show that CSDS could affect the spontaneous locomotor activity of mice, but not the grip strength and forced locomotor ability. Chemogenetic activation of VTA-DA neurons alleviated CSDS-induced hypolocomotion. Subsequently, quantitative whole-brain mapping revealed decreased projections from VTA-DA neurons to substantia nigra pars reticulata (SNr) after CSDS treatment. Optogenetic activation of dopaminergic projection from VTA to SNr with the stimulation of phasic firing, but not tonic firing, could significantly increase the locomotor activity of mice. Moreover, chemogenetic activation of VTA-SNr dopaminergic circuit in CSDS mice could also rescued the decline of locomotor activity. Taken together, our data suggest that the VTA-SNr dopaminergic projection mediates CSDS-induced hypolocomotion, which provides a theoretical basis and potential therapeutic target for MDD.






Similar content being viewed by others
Data Availability
Data will be made available on reasonable request.
All animal handling and experiments were performed in accordance with NIH guidelines and reviewed by the Ethics Committees of Huazhong University of Science and Technology (HUST).
References
Yoon G, Petrakis IL, Krystal JH (2019) Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiat 76(3):337–338. https://doi.org/10.1001/jamapsychiatry.2018.3990
Knowland D, Lilascharoen V, Pacia CP, Shin S, Wang EH, Lim BK (2017) Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell 170(2):284-2297 e218. https://doi.org/10.1016/j.cell.2017.06.015
Nestler EJ, Hyman SE (2010) Animal models of neuropsychiatric disorders. Nat Neurosci 13(10):1161–1169. https://doi.org/10.1038/nn.2647
Kalueff AV, Fox MA, Gallagher PS, Murphy DL (2007) Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav 6(4):389–400. https://doi.org/10.1111/j.1601-183X.2006.00270.x
Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin JS, Schorscher-Petcu A, Langford DJ, Bennett GJ (2010) Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain 6:34. https://doi.org/10.1186/1744-8069-6-34
Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F (1998) Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience 82(1):147–159. https://doi.org/10.1016/s0306-4522(97)00282-0
Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011) A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6(8):1183–1191. https://doi.org/10.1038/nprot.2011.361
Douma EH, de Kloet ER (2020) Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci Biobehav Rev 108:48–77. https://doi.org/10.1016/j.neubiorev.2019.10.015
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC et al (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493(7433):532–536. https://doi.org/10.1038/nature11713
Matsumoto M, Hikosaka O (2009) Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459(7248):837–841. https://doi.org/10.1038/nature08028
Howe MW, Dombeck DA (2016) Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535(7613):505–510. https://doi.org/10.1038/nature18942
Morales M, Margolis EB (2017) Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18(2):73–85. https://doi.org/10.1038/nrn.2016.165
Grace AA, Floresco SB, Goto Y, Lodge DJ (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30(5):220–227. https://doi.org/10.1016/j.tins.2007.03.003
Mohebi A, Pettibone JR, Hamid AA, Wong JT, Vinson LT, Patriarchi T, Tian L, Kennedy RT et al (2019) Dissociable dopamine dynamics for learning and motivation. Nature 570(7759):65–70. https://doi.org/10.1038/s41586-019-1235-y
Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L (2015) Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162(3):622–634. https://doi.org/10.1016/j.cell.2015.07.015
Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD (2012) The mouse forced swim test. J Vis Exp 59:e3638. https://doi.org/10.3791/3638
Mei Y, Monteiro P, Zhou Y, Kim JA, Gao X, Fu Z, Feng G (2016) Adult restoration of Shank3 expression rescues selective autistic-like phenotypes. Nature 530(7591):481–484. https://doi.org/10.1038/nature16971
Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, Guo W, Kang J et al (2015) Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci 18(3):423–434. https://doi.org/10.1038/nn.3930
Davies JE, Wang L, Garcia-Oroz L, Cook LJ, Vacher C, O’Donovan DG, Rubinsztein DC (2005) Doxycycline attenuates and delays toxicity of the oculopharyngeal muscular dystrophy mutation in transgenic mice. Nat Med 11(6):672–677. https://doi.org/10.1038/nm1242
Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernandez G et al (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537(7620):357–362. https://doi.org/10.1038/nature19325
Stauffer WR, Lak A, Yang A, Borel M, Paulsen O, Boyden ES, Schultz W (2016) Dopamine neuron-specific optogenetic stimulation in rhesus macaques. Cell 166(6):1564-1571 e1566. https://doi.org/10.1016/j.cell.2016.08.024
Beier KT, Kim CK, Hoerbelt P, Hung LW, Heifets BD, DeLoach KE, Mosca TJ, Neuner S et al (2017) Rabies screen reveals GPe control of cocaine-triggered plasticity. Nature 549(7672):345–350. https://doi.org/10.1038/nature23888
Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S et al (2015) Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524(7563):88–92. https://doi.org/10.1038/nature14600
Wu X, Liu BJ, Ji S, Wu JF, Xu CQ, Du YJ, You XF, Li B et al (2015) Social defeat stress promotes tumor growth and angiogenesis by upregulating vascular endothelial growth factor/extracellular signal-regulated kinase/matrix metalloproteinase signaling in a mouse model of lung carcinoma. Mol Med Rep 12(1):1405–1412. https://doi.org/10.3892/mmr.2015.3559
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A et al (2013) Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493(7433):537–541. https://doi.org/10.1038/nature11740
Macedo GC, Morita GM, Domingues LP, Favoretto CA, Suchecki D, Quadros IMH (2018) Consequences of continuous social defeat stress on anxiety- and depressive-like behaviors and ethanol reward in mice. Horm Behav 97:154–161. https://doi.org/10.1016/j.yhbeh.2017.10.007
Blandini F, Nappi G, Tassorelli C, Martignoni E (2000) Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog Neurobiol 62(1):63–88
Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T (2003) Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 119(1):293–308. https://doi.org/10.1016/s0306-4522(03)00095-2
Nestler EJ, Carlezon WA Jr (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59(12):1151–1159. https://doi.org/10.1016/j.biopsych.2005.09.018
Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324(5930):1080–1084. https://doi.org/10.1126/science.1168878
Billes SK, Cowley MA (2008) Catecholamine reuptake inhibition causes weight loss by increasing locomotor activity and thermogenesis. Neuropsychopharmacology 33(6):1287–1297. https://doi.org/10.1038/sj.npp.1301526
Redolat R, Vidal J, Gomez MC, Carrasco MC (2005) Effects of acute bupropion administration on locomotor activity in adolescent and adult mice. Behav Pharmacol 16(1):59–62. https://doi.org/10.1097/00008877-200502000-00007
Sachs BD, Ni JR, Caron MG (2014) Sex differences in response to chronic mild stress and congenital serotonin deficiency. Psychoneuroendocrinology 40:123–129. https://doi.org/10.1016/j.psyneuen.2013.11.008
Duncan N, Zimmer-Gembeck MJ, Furman W (2019) Sexual harassment and appearance-based peer victimization: unique associations with emotional adjustment by gender and age. J Adolesc 75:12–21. https://doi.org/10.1016/j.adolescence.2019.06.016
LeGates TA, Kvarta MD, Thompson SM (2019) Sex differences in antidepressant efficacy. Neuropsychopharmacology 44(1):140–154. https://doi.org/10.1038/s41386-018-0156-z
Seibenhener ML, Wooten MC (2015) Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 96:e52434. https://doi.org/10.3791/52434
Pan HQ, Zhang WH, Liao CZ, He Y, Xiao ZM, Qin X, Liu WZ, Wang N, Zou JX, Liu XX, Pan BX (2020) Chronic stress oppositely regulates tonic inhibition in Thy1-expressing and non-expressing neurons in amygdala. Front Neurosci 14:299. https://doi.org/10.3389/fnins.2020.00299
Acknowledgements
The authors wish to acknowledge Dr. Xutao Zhu, Dr. Sen Jin (Center for Brain Science, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, Wuhan, 430071 China), and Dr. Manfei Deng (Department of Pathophysiology, School of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, 430030, P. R. China) for their technical assistance with this project.
Funding
This work was supported financially by grants from National Natural Science Foundation of China (Nos. 31871073, 31571044 to B.T., and No. 31600821 to P.Z.), Program for New Century Excellent Talents in University (No. NCET-10–0415 to B.T.), and the Fundamental Research Funds for the Central Universities (HUST: 2019kfyXJJS081 to P.Z.).
Author information
Authors and Affiliations
Contributions
F.H.: investigation, methodology, data curation, formal analysis, writing—original draft, visualization. P.Z.: conceptualization, methodology, data curation, formal analysis, writing—review and editing, visualization, project administration, funding acquisition. Q.Z.: investigation, methodology, formal analysis, validation, visualization. G.J.Q.: validation, data curation, formal analysis, visualization. H.W.C.: investigation, formal analysis, validation, data curation. T.X.L.: investigation, validation, data curation. M.L.: investigation, validation, data curation. J.Z.L.: investigation, formal analysis. J.E.L.: investigation, formal analysis. J.M.: conceptualization, resources, supervision. B.T.: conceptualization, methodology, resources, writing—review and editing, visualization, supervision, project administration, funding acquisition.
Corresponding authors
Ethics declarations
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Feng He and Pei Zhang contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, F., Zhang, P., Zhang, Q. et al. Dopaminergic Projection from Ventral Tegmental Area to Substantia Nigra Pars Reticulata Mediates Chronic Social Defeat Stress–Induced Hypolocomotion. Mol Neurobiol 58, 5635–5648 (2021). https://doi.org/10.1007/s12035-021-02522-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02522-7