Abstract
Magnesium–aluminum hydrotalcite (HT) supported gold catalyst (1 wt% Au/HT) was prepared by the colloid-deposition method and characterized by inductively coupled plasma atomic emission spectroscopy (ICP-AES), X-ray diffraction (XRD), nitrogen adsorption, transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and Hammett indicator method. The effects of calcination temperature of catalyst and support on the catalytic performance of aerobic oxidative esterification of 1,3-propanediol with methanol to methyl 3-hydroxypropionate (3-HPM) under base-free condition were studied. The results showed that the conversion of 1,3-propanediol and the selectivity of 3-HPM increased first and then decreased with the increase of calcination temperature of catalyst and support. The optimal calcination temperature of catalyst and support is 150 °C. Under the optimum preparation conditions, the conversion of 1,3-propanediol and the selectivity to 3-HPM are 96.2% and 94.9%, respectively. In addition, the 1 wt% Au/HT catalyst could be effectively recovered by calcining at 150 °C in air atmosphere, and the performance of the catalyst does not decrease significantly. The performance of the catalyst is higher than that reported previously. The structure of magnesium–aluminum hydrotalcite with appropriate density and medium strength base sites and small metallic Au particles are favorable for the selective oxidative esterification of 1,3-propanediol to 3-HPM.
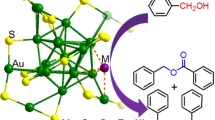
Graphic abstract
A 1 wt% magnesium–aluminum hydrotalcite supported gold catalyst (1 wt% Au/HT) was prepared by the colloid-deposition method. The calcination temperature plays an important role for the catalytic performance and the higher catalytic activity and product selectivity is due to the synergistic effect between the support structure with appropriate density and medium strength base sites and the smaller metallic gold particles.








Similar content being viewed by others
References
Corma A, Iborra S, Velty A (2007) Chemical routes for the transformation of biomass into chemicals. Chem Rev 107:2411–2502. https://doi.org/10.1002/chin.200736263
Zhao G, Gao E, Wan Q (2019) Structure-activity relationships of Au/Al2O3 catalyst for the selective oxidative esterification of 1,3-propanediol and methanol. ChemistrySelect 4(43):12479–12490. https://doi.org/10.1002/slct.201903059
Saxena RK, Anand P, Saran S (2009) Microbial production of 1,3-propanediol: Recent developments and emerging opportunities. Biotechnol Adv 27(6):895–913. https://doi.org/10.1016/j.biotechadv.2009.07.003
De Souza EA, Rossi DM, Zachia Ayub MA (2014) Bioconversion of residual glycerol from biodiesel synthesis into 1,3-propanediol using immobilized cells of Klebsiella pneumoniae BLh-1. Renewable Energy 72:253–257. https://doi.org/10.1016/j.renene.2014.07.030
Wang X, Zhao G, Zou H (2011) The base-free and selective oxidative transformation of 1,3-propanediol into methyl esters by different Au/CeO2 catalysts. Green Chem 13(10):2690–2695. https://doi.org/10.1039/c1gc15588a
Kurian JV (2005) A new polymer platform for the future — Sorona® from Corn Derived 1,3-Propanediol. J Polym Environ 13(2):159–167. https://doi.org/10.1007/s10924-005-2947-7
Hayashi T, Inagaki T, Itayama N (2006) Selective oxidation of alcohol over supported gold catalysts: methyl glycolate formation from ethylene glycol and methanol. Catal Today 117(1–3):210–213. https://doi.org/10.1016/j.cattod.2006.06.045
Menegazzo F, Signoretto M, Marchese D (2015) Structure–activity relationships of Au/ZrO2 catalysts for 5-hydroxymethylfurfural oxidative esterification: Effects of zirconia sulphation on gold dispersion, position and shape. J Catal 326:1–8. https://doi.org/10.1016/j.jcat.2015.03.006
Mullen GM, Evans EJJ, Sabzevari I (2017) Water influences the activity and selectivity of ceria-supported gold catalysts for oxidative dehydrogenation and esterification of ethanol. ACS Catal 7(2):1216–1226. https://doi.org/10.1021/acscatal.6b02960
Taarning E, Theilgaard Madsen A, Marchetti JM (2008) Oxidation of glycerol and propanediols in methanol over heterogeneous gold catalysts. Green Chem 10(4):408–414. https://doi.org/10.1039/b714292g
Menegazzo F, Fantinel T, Signoretto M (2014) On the process for furfural and HMF oxidative esterification over Au/ZrO2. J Catal 319:61–70. https://doi.org/10.1016/j.jcat.2014.07.017
Miyamura H, Kobayashi S (2014) Tandem oxidative processes catalyzed by polymer-incarcerated multimetallic nanoclusters with molecular oxygen. Accumulation Chemistry Resolution 47(4):1054–1066. https://doi.org/10.1021/ar400224f
Zuo C, Tian Y, Zheng Y (2019) One step oxidative esterification of methacrolein with methanol over Au-CeO2/γ-Al2O3 catalysts. Catal Commun 124:51–55. https://doi.org/10.1016/j.catcom.2019.03.002
Casanova O, Iborra S, Corma A (2009) Biomass into chemicals: One pot-base free oxidative esterification of 5-hydroxymethyl-2-furfural into 2,5-dimethylfuroate with gold on nanoparticulated ceria. J Catal 265(1):109–116. https://doi.org/10.1016/j.jcat.2009.04.019
Sankar M, He Q, Engel R, V, (2020) Role of the support in gold-containing nanoparticles as heterogeneous catalysts. Chem Rev 120(8):3890–3938. https://doi.org/10.1021/acs.chemrev.9b00662
Sullivan JA, Burnham S (2015) The use of alkaline earth oxides as pH modifiers for selective glycerol oxidation over supported Au catalysts. Renewable Energy 78:89–92. https://doi.org/10.1016/j.renene.2014.12.068
Purushothaman RKP, van Haveren J, Melian-Cabrera I (2014) Base-free, one-pot chemocatalytic conversion of glycerol to methyl lactate using supported gold catalysts. Chemsuschem 7(4):1140–1147. https://doi.org/10.1002/cssc.201301105
Nishimura S, Takagaki A, Ebitani K (2013) Characterization, synthesis and catalysis of hydrotalcite-related materials for highly efficient materials transformations. Green Chem 15(8):2026–2042. https://doi.org/10.1039/c3gc40405f
Baskaran T, Christopher J, Sakthivel A (2015) Progress on layered hydrotalcite (HT) materials as potential support and catalytic materials. RSC Adv 5(120):98853–98875. https://doi.org/10.1039/c5ra19909c
Liu P, Guan Y, van Santen RA (2011) Aerobic oxidation of alcohols over hydrotalcite-supported gold nanoparticles: the promotional effect of transition metal cations. Chem Commun 47(41):11540–11542. https://doi.org/10.1039/c1cc15148g
Mobley JK, Crocker M (2015) Catalytic oxidation of alcohols to carbonyl compounds over hydrotalcite and hydrotalcite-supported catalysts. RSC Adv 5:65780–65797. https://doi.org/10.1039/C5RA11254K
Mitsudome T, Noujima A, Mizugaki T (2009) Supported gold nanoparticles as a reusable catalyst for synthesis of lactones from diols using molecular oxygen as an oxidant under mild conditions. Green Chem 11(6):793–797. https://doi.org/10.1039/b900576e
Liu P, Li C, Hensen EJM (2012) Efficient tandem synthesis of methyl esters and imines by using versatile hydrotalcite-supported gold nanoparticles. Chem Eur J 18(38):12122–12129. https://doi.org/10.1002/chem.201202077
Wei H, Li J, Yu J (2015) Gold nanoparticles supported on metal oxides as catalysts for the direct oxidative esterification of alcohols under mild conditions. Inorg Chim Acta 427:33–40. https://doi.org/10.1016/j.ica.2014.11.024
Zhang Z, Xu S, Zeng H (2016) Influence of calcination temperature on the microstructure and catalytic performance of Mg/Al hydrotalcites catalysts for alcoholysis of propylene oxide. J Nanosci Nanotechnol 16(12):12677–12683. https://doi.org/10.1166/jnn.2016.13780
Pinthong P, Praserthdam P, Jongsomjit B (2019) Effect of calcination temperature on Mg-Al Layered Double Hydroxides (LDH) as promising catalysts in oxidative dehydrogenation of ethanol to acetaldehyde. J Oleo Sci 68(1):95–102. https://doi.org/10.5650/jos.ess18177
Cavani F, Trifirb F, Vaccari A (1991) Hydrotalcite cite-type anionic clays: preparation, properites and applicatioms. Catal Today 11:173–301. https://doi.org/10.1002/chin.199212317
Ebitani K, Motokura K, Mori K (2006) Reconstructed hydrotalcite as a highly active heterogeneous base catalyst for carbon-carbon bond formations in the presence of water. J Org Chem 71:5440–5447. https://doi.org/10.1021/jo060345l
Xu ZP, Stevenson G, Lu C-Q (2006) Dispersion and size control of layered double hydroxide nanoparticles in aqueous solutions. J Chem Phys 110:16923–16929. https://doi.org/10.1021/jp062281o
Kotionova T, Lee C, Miedziak PJ (2012) Oxidative Esterification of Homologous 1,3-Propanediols. Catal Lett 142(9):1114–1120. https://doi.org/10.1007/s10562-012-0872-7
Ke Y-H, Qin X-X, Liu C-L (2014) Oxidative esterification of ethylene glycol in methanol to form methyl glycolate over supported Au catalysts. Catal Sci Technol 4(9):3141–3150. https://doi.org/10.1039/c4cy00556b
Tang R, Tian Y, Qiao Y (2016) Bifunctional base catalyst for vacuum residue cracking gasification. Fuel Process Technol 153:1–8. https://doi.org/10.1016/j.fuproc.2016.07.022
Redondo N, Dieuzeide ML, Amadeo N (2020) Acid removal from crude oils by catalytic esterification naphthenic acid catalize by Mg/Al hydrotalcite. Catal Today 353:82–87. https://doi.org/10.1016/j.cattod.2019.09.051
Fang S, Xia Y, Lv K (2016) Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl Catal B 185:225–232. https://doi.org/10.1016/j.apcatb.2015.12.025
Liu M, Fan G, Yu J (2018) Defect-rich Ni-Ti layered double hydroxide as a highly efficient support for Au nanoparticles in base-free and solvent-free selective oxidation of benzyl alcohol. Dalton Trans 47(15):5226–5235. https://doi.org/10.1039/c7dt04229a
Foster DM, Pavloudis T, Kioseoglou J, Palmer RE (2019) Atomic-resolution imaging of surface and core melting in individual size-selected Au nanoclusters on carbon. Nature Communication 10(1):2583. https://doi.org/10.1038/s41467-019-10713-z
Sahua N, Paridaa KM, Tripathib AK, Kambleb VS (2011) Low temperature CO adsorption and oxidation over Au/rare earth-TiO2 nanocatalysts. Appl Catal A 399(1–2):110–116. https://doi.org/10.1016/j.apcata.2011.03.052
Xin JY, Lin K, Wang Y, Xia CG (2014) Methanobactin-mediated synthesis of gold nanoparticles supported over Al2O3 toward an efficient catalyst for glucose oxidation. Int J Mol Sci 15(12):21603–21620. https://doi.org/10.3390/ijms151221603
Zhang X, Wang H, Xu B (2005) Remarkable nanosize effect of zirconia in Au@ZrO2 catalyst for CO oxidation. J Phys Chem B 109:9678–9683. https://doi.org/10.1021/jp050645r
Tian Y, Zhou H, Qiao Y, Tang R, Zhao G, Leng G (2017) In-situ reduction of Cu(CH3COO)2 to prepare π-complexation adsorbent for propylene/propane separation by slurry bed. Sep Sci Technol 52(12):1959–1966. https://doi.org/10.1080/01496395.2017.1310238
Mackenzie KJD, Meinhold RH, Sherriff BL (1993) 27Al and 25Mg solid-state magic-angle spinning nuclear magnetic resonance study of hydrotalcite and its thermal decomposition sequence. J Mater Chem 3(12):1263–1269. https://doi.org/10.1039/jm9930301263
Tang R, Wang S, Che Y (2017) Adjustment of the product distribution over a bifunctional Ca12Al14O33-supported MnOx catalyst from cracking gasification of the petroleum residue. Energy Fuels 31(6):5995–6003. https://doi.org/10.1021/acs.energyfuels.7b00600
Take J-I, Kikuchi N, Yoneda Y (1971) Base-strength distribution studies of solid-base surfaces. J Catal 21(2):164–170. https://doi.org/10.1016/0021-9517(71)90134-5
Hammett L, Deyrup A (1932) A series of simple basic indicators II Some applications to solutions in formic acid. J Am Chem Soc 54(11):4239–4247. https://doi.org/10.1021/ja01346a015
Shen JT, Mai HuC (1998) Structural and surface acid-base properties of hydrotalcite-derived MgAlO oxides calcined at varying temperatures. J Solid State Chem 137(2):295–301. https://doi.org/10.1006/jssc.1997.7739
Zeng H-Y, Xu S, Liao M-C (2014) Activation of reconstructed Mg/Al hydrotalcites in the transesterification of microalgae oil. Appl Clay Sci 91–92:16–24. https://doi.org/10.1016/j.clay.2014.02.003
Song J, Yu G, Li X, Yang X, Zhang W, Yan W, Liu G (2018) Oxidative coupling of alcohols and amines to an imine over Mg-Al acid-base bifunctional oxide catalysts. Chin J Catal 39(2):309–318. https://doi.org/10.1016/s1872-2067(17)63006-7
Brett GL, Miedziak PJ, Knight DW, Taylor SH, Hutchings GJ (2013) Gold-Based Nanoparticulate Catalysts for the Oxidative Esterification of 1,4-Butanediol to Dimethyl Succinate. Top Catal 57(6–9):723–729. https://doi.org/10.1007/s11244-013-0229-5
Fang W, Chen J, Zhang Q, Deng W, Wang Y (2011) Hydrotalcite-supported gold catalyst for the oxidant-free dehydrogenation of benzyl alcohol: studies on support and gold size effects. Chemistry 17(4):1247–1256. https://doi.org/10.1002/chem.201002469
Lin Y, Lu G-P, Zhao X, Cao X, Yang L, Zhou B, Zhong Q, Chen Z (2020) Porous cobalt@N-doped carbon derived from chitosan for oxidative esterification of 5-Hydroxymethylfurfural: The roles of zinc in the synthetic and catalytic process. Mol Catal. https://doi.org/10.1016/j.mcat.2019.110695
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21206185), Qingdao Postdoctoral Applied Research Project (Grant No. 2015202), Scientific Research Foundation of Shandong University of Science and Technology for Recruited Talents (Grant No. 2016RCJJ006), Innovative training program for College Students (Grant No. 202010424079), the fund of the Key Laboratory of Catalysis and Energy Materials Chemistry of Ministry of Education & Hubei Key Laboratory of Catalysis and Materials Science (Grant No. CHCL20003), science and technology research guiding plan project of China Coal Industry Association (Grant No. MTKJ 2016-267).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wan, Q., Wang, X., Zhao, B. et al. Influence of calcination temperature on the cooperative catalysis of base sites and gold nanoparticles on hydrotalcite-supported gold materials for the base-free oxidative esterification of 1, 3-propanediol with methanol to methyl 3-hydroxypropionate. Reac Kinet Mech Cat 134, 109–125 (2021). https://doi.org/10.1007/s11144-021-02042-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02042-4