Published online Jul 26, 2021. doi: 10.4252/wjsc.v13.i7.737
Peer-review started: February 7, 2021
First decision: May 5, 2021
Revised: May 13, 2021
Accepted: June 15, 2021
Article in press: June 15, 2021
Published online: July 26, 2021
The mitochondrial unfolded protein response (UPRmt) is an evolutionarily conserved adaptive mechanism for improving cell survival under mitochondrial stress. Under physiological and pathological conditions, the UPRmt is the key to maintaining intracellular homeostasis and proteostasis. Important roles of the UPRmt have been demonstrated in a variety of cell types and in cell development, metabolism, and immune processes. UPRmt dysfunction leads to a variety of pathologies, including cancer, inflammation, neurodegenerative disease, metabolic disease, and immune disease. Stem cells have a special ability to self-renew and differentiate into a variety of somatic cells and have been shown to exist in a variety of tissues. These cells are involved in development, tissue rene
Core Tip: Mitochondrial unfolded protein response (UPRmt) is a newly discovered equilibrium stress mechanism of mitochondria in the stressed cells to maintain the stressed proteins. Stem cells are a group of cells that have an infinite or eternal ability to self-renew and affect cell differentiation, aging, cancer, etc. Here we discuss the potential significance of UPRmt in stem cells, with an aim to provide a new theoretical basis and therapeutic target for cell differentiation, senescence, and some diseases.
- Citation: Gu LF, Chen JQ, Lin QY, Yang YZ. Roles of mitochondrial unfolded protein response in mammalian stem cells. World J Stem Cells 2021; 13(7): 737-752
- URL: https://www.wjgnet.com/1948-0210/full/v13/i7/737.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i7.737
Mitochondria are double-membrane-bound organelles composed of four compartments: The outer and inner membranes, the intermembrane space, and the matrix[1]. While mitochondria provide some metabolic advantages for eukaryotic cells, they may be detrimental in other ways[2]. For example, reactive oxygen species (ROS) produced by mitochondria damage the nucleus, cytoplasm, and mitochondria, leading to mutations, aging, and diseases[3]. The implication is that mitochondria may be significant to mammalian stem cell functions[4].
The normal functions of mitochondria (mitochondrial homeostasis) are very important for cells and individuals. Therefore, to maintain mitochondrial homeostasis, organisms have evolved a series of mitochondrial quality control pathways, one of which is the mitochondrial unfolded protein response (UPRmt)[5]. UPRmt is an adaptive transcriptional response that was initially described as a mechanism by which cells maintain mitochondrial protein homeostasis during mitochondrial dysfunction. The program includes genes that promote mitochondrial protein homeostasis and defective organelle recovery[6]. It transfers signals that are helpful for maintaining the homeostasis of mitochondria-related proteins and cell survival from the mitochon
The mitochondrial UPRmt pathways in mammalian cells are largely unknown. The following information on UPRmt pathways has been discovered thus far[9-11]:(1) The UPRmt pathway is induced by protein accumulation in the mitochondrial matrix; (2) The UPRmt pathway is induced by protein accumulation in the mitochondrial membrane gap; (3) The UPRmt pathway is regulated by the activating transcription factor associated with stress-1 (ATFS-1) homologous gene ATF5; and (4) The UPRmt pathway is regulated by the heat shock factor 1-single-stranded DNA-binding protein 1 (HSF1-SSBP1) complex under heat stress.
Through these four pathways, the mitochondrial load is ameliorated by the following four methods: (1) When a large number of misfolded proteins accumulate in mitochondria, c-Jun N-terminal kinase 2 (JNK2) is activated, which promotes the phosphorylation of c-Jun[7]. Activated c-Jun binding at an AP-1 binding site induces CCAAT-enhancer binding protein (C/EBP) homologous protein (CHOP) and C/EBP expression. CHOP and C/EBP proteins form dimers that act as transcription factors by binding the promoter of UPRmt-related genes, thereby inducing the expression of mitochondrial heat shock proteins and proteases[12,13]; (2)When a large number of misfolded proteins accumulate in the mitochondrial membrane gap, a large number of ROS produced by mitochondria can activate AKT kinase. Phosphorylated AKT kinase promotes the estrogen receptor (ER)activity, induces the NRF1 gene to promote biosynthesis in mitochondria, and induces the expression of mitochondrial protease high temperature requirement protein A2 to restore mitochondrial function[11]; (3) ATF5 functions similarly to the ATFS-1 pathway induced in nematodes[14]. ATF5 induces high expression of mitochondrial heat shock proteins (HSP60 and mtHSP70), the mitochondrial protease Lon, and the antimicrobial peptide HD-5 in mammalian cells and promotes cell proliferation and mitochondrial function recovery under stress[14]; and (4) Under the condition of heat stress, the mitochondrial single-stranded DNA-binding proteins SSBP1 and HSF1 jointly regulate the UPRmt pathway. Under the condition of heat stress or other protein-induced toxicity, mitochondrial membrane potential is decreased, and SSBP1 is discharged from the mitochondria by means of the ANT- VDAC1 complex. After binding with HSF1, SSBP1 is transported to the nucleus, and through the recruitment of chromatin regulators, open chromatin is formed to drive high levels of transcription. The HSF1-SSBP1 complex is associated with the expression of nuclear and cytoplasmic molecular chaperones (HSP70, etc.) and mitochondrial molecular chaperones (HSP60 and HSP10)[15].
Although the precise pathways and marker molecules of the UPRmt are not known, ATF4, CHOP, HSP60, HSP10, caseinolytic protease proteolytic subunit (CLPP), LON peptidase 1, mitochondrial (Lonp1), JNK2, c-Jun, C/EBP protein, Sirtuin3 (SIRT3), and ATF5 have been found to be important regulators of the UPRmt in mammals[16-19]. Most of these proteins are molecular chaperones and proteolytic enzymes that promote the correct folding of mitochondrial proteins, and the UPRmt is a protective program activated by the transcriptome of gene groups that include molecular chaperone and proteolytic enzyme genes with transcription initiated by mitochondria to maintain the homeostasis of their internal proteins[20].
Stem cells produce all the cells and build tissue structure in the body, and they are critical for maintaining tissue homeostasis. The characteristics of stem cells are heterogeneity and plasticity. Understanding the properties of stem cells improves our ability to maintain tissue homeostasis[21].
However, it is not clear whether the UPRmt is associated with mitochondrial dysfunction, protein homeostasis, oxidative stress, and/or autophagy in stem cells. Therefore, studying the link between the UPRmt and stem cells is crucial to understanding the development of human beings. UPRmt activation in stem cells may act as a sentinel against mitochondrial damage[22].
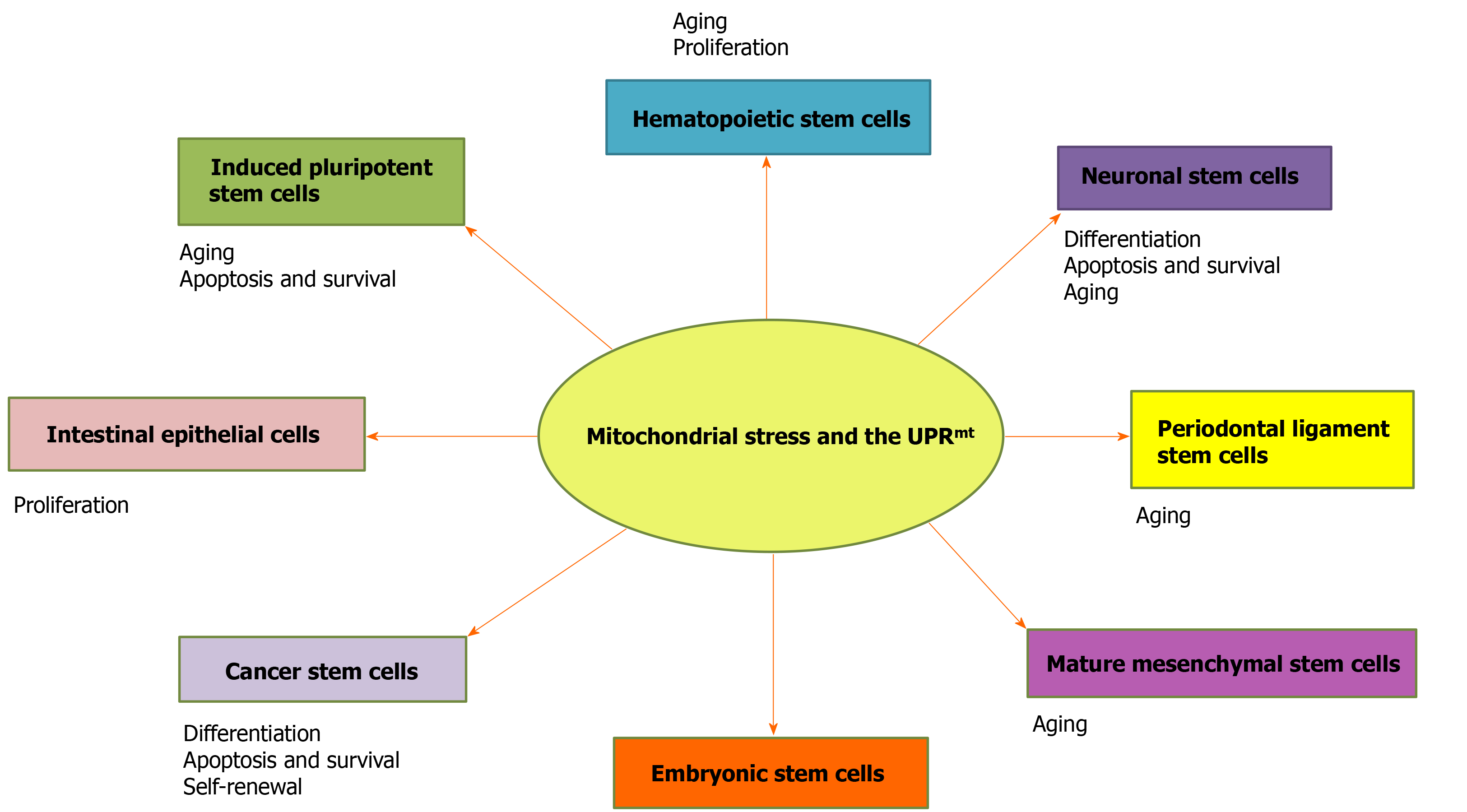
In this review, we discuss the roles of the UPRmt in stem cell proliferation, differentiation, and aging in mammals (Figure 1), and the role of UPRmt-regulated genes in stem cells is summarized (Table 1). This work provides a novel perspective on the maintenance of cell homeostasis, life extension, stem cell therapy, and cancer.
| Modulator | Functions | Ref. |
| ATF5 | A transcription factor that is highly expressed in undifferentiated neural progenitor cells/stem cells and in a variety of human cancers, including gliomas. | [132] |
| HD | Antimicrobial peptide. | [14] |
| CHOP | A transcription factor that is upregulated in response to ER stress and the mitochondrial unfolded protein response, and results in impaired wound healing and reduced IEC proliferation. | [133] |
| LONP1 | The mitochondrial matrix proteases. | [134] |
| HSP60 | A mitochondrion-localized chaperone that is responsible for maintenance of the mitochondrial proteome and is upregulated as a part of the mitochondrial unfolded protein response. | [135] |
| AKT | A serine-threonine kinase that maintains stem cells by promoting viability and proliferation. | [136] |
| CLPP | A mitochondrial matrix protease and mitochondrion-localized chaperone that is responsible for maintenance of the mitochondrial proteome and is upregulated as a part of the mitochondrial unfolded protein response. | [134,135] |
| SIRT3 | SIRT3 has been reported to be a tumor suppressor and mitochondrial stress stimulates SIRT3 activity, raising the possibility that SIRT3 plays a dual role in tumorigenesis. | [16] |
| SIRT7 | The strongest deacetylase activity, found in the nucleus, which can result in increased mitochondrial stress and increased tendency to proliferate. | [137] |
| JNK | New target protein in mitochondrial dependent apoptosis. | [84] |
| HSP70 | Mitochondrial chaperone. | [14] |
| HSP60 | Stem cells without HSP60 lose their ability to self-renew. | [71] |
Embryonic stem cells (ESCs) are derived from the blastocyst inner cell mass (ICM). In vivo, ESCs differentiate into three primary germ layers: The ectoderm, endoderm, and mesoderm. In vitro differentiation of human ESCs can help with research into certain diseases, screenings for drug discovery, and identifying cells for regenerative use[22]. Mitochondria are essential for maintaining the properties of ESCs and regulate their subsequent differentiation into different cell lineages[23,24]. Studies have shown that the proliferation and differentiation of ESCs depend on the normal function of mitochondria[25].
AKT1 is activated and transferred to mitochondria after stimulation by growth factors in ESCs. Genes that promote the proliferation and survival of ESCs are upregulated, while genes that promote differentiation are downregulated[26]. CHOP activates UPRmt-related genes, and its expression is induced via activation of Jun, which is mediated by c-Jun N-terminal kinase 2[12,13]. Interestingly, the growth of ESCs was not affected by c-Jun deficiency, but c-Jun-deficient fibroblasts was arrested in the G1 phase of the cell cycle, suggesting that the effect of c-Jun on cell proliferation is cell type-dependent[27].Does the absence of c-Jun in ESCs affect fibroblast differentiation? Pharmacological and genetic evidence supports the supposition that c-Jun plays an important role in neural induction of ESCs[28]. The effect of UPRmt-induced c-Jun in intestinal epithelial cells (IECs) depends on the activity of the mitochondrial protease CLPP and cytoplasmic kinase PKR[29]. Indeed, c-Jun is a vital regulatory factor of the UPRmt[16-19].
Studies have shown that ESCs have only a small number of immature mitochondria, and additional mitochondria with mature characteristics, such as fully developed cristae, dense matrix, and higher oxidation capacity, are evident during the process of differentiation[30,31]. It is speculated that the UPRmt may be involved in the differentiation process of ESCs.
HSP60 is a mitochondrial protein important for folding key proteins after its introduction into the mitochondrion. In the heart, the binding of HSP60 to Bax in the cytoplasm plays a key role in the regulation of apoptosis[32]. Overexpression of HSP60 increased the expression of the antiapoptotic Bcl-2 gene and decreased the level of the proapoptotic Bax protein[33]. The apoptosis induced by mycotoxin citrinin in ESCs was induced by ROS production, which also increased the cytoplasmic free calcium level, intracellular nitric oxide production, the Bax/Bcl-2 ratio, the loss of mitochon
However, evidence showing that mitochondrial homeostasis regulates the pluripotency of ESCs remains to be seen[37], and the exact role of the UPRmt needs to be explored in future studies.
Germline stem cells (GSCs) were categorized into female GSCs (FGSCs) and spermatogonial stem cells (SSCs). Research on human GSCs was originally on SSCs. The role of the UPRmt in SSCs has rarely been reported, but the role of the UPRmt in sperm has been discussed. Male mammals are able to continuously produce sperm due to the self-renewal and differentiation abilities of SSCs[38]. The conventional wisdom is that the number of follicles in mammals ceases to increase once a primal follicular pool is formed after birth. With development, maturation, and atresia, follicles are constantly depleted, ovarian function gradually declines, and women gradually enter menopause[39]. However, the discovery of FGSCs, which are derived from primitive germline cells and have the ability to differentiate into oocytes in a directional manner, is expected to lead to the replenishment of depleting primal follicular pools[40]. Although the role of the UPRmt in FGSCs has rarely been reported, the roles of the UPRmt in follicular development and atresia have been discussed.
The differentiation of GSCs is characterized by gradual changes in the structure of multiple organelles, among which the mitochondrion plays unique roles[41]. During oocyte maturation and senescence, mitochondrial aggregation is related to germ cell formation and epigenetic regulation[42].
More recently, the absence of CLPP, a mitochondrial stress response gene, has been shown to cause female infertility and accelerate ovarian follicular failure[43]. CLPP mutation was observed in Perrault syndrome, which is associated with defects in human ovaries[44]. The study of CLPP-null mice (using CLPP gene ablation) showed complete female and male infertility[45]. CLPP deletion caused selective profound vulnerability of specific cells in testes and ovaries. Therefore, mutations in CLPP may be related to the survival of GSCs. C/EBPβ is a transcriptional activator of PGC-1α in developing embryos, whereas CHOP blocks the DNA-binding ability of C/EBPβ by forming a CHOP/C/EBP heterodimer and repressing PGC-1α expression[46]. In mouse GC culture, upregulated CHOP expression was induced, leading to apoptosis. ATF4 and CHOP expression was higher in the GCs of goats with follicular atresia[46]. This suggests that the UPRmt may be related to GC apoptosis, which damages the viability of oocytes and embryos.
The UPRmt marker c-Jun is involved in a number of mammalian male reproductive processes, including spermatogenesis, sperm maturation and activation, and acrosomal responses prior to oocyte fertilization[47]. Therefore, the role of the UPRmt in GSCs in infancy might be explored in future studies.
Hematopoietic stem cells (HSCs) are adult stem cells in the blood system. HSCs form a heterogeneous population with the capacity for long-term self-renewal and the potential to differentiate into various mature blood cells. HSCs, similar to many types of stem cells, are particularly vulnerable to damage from ROS, and the damage may be transmitted to progenitor cells, leading to various pathological conditions[48,49]. The main sources of ROS in cells are mitochondria[50].
A study showed that the UPRmt is activated upon HSC transition from quiescence to proliferation[51]. Remodeling the activity of SIRT7, a component of the UPRmt, translates into a reduction in quiescence, and higher SIRT7 activation can rescue the reduced regenerative capacity of aged HSCs[17]. These findings suggest that inhibition of SIRT7 can enhance the biological generation of mitochondria and activate the UPRmt, thereby reducing its quiescence and differentiation ability[4]. Thus, SIRT7-mediated UPRmt may be important for cells that experience bursts of mitochondrial biogenesis and transition between growth states with markedly different bioenergetic demands and proliferative potentials, such as stem cells[52]. During osteogenic differentiation, SIRT7 is downregulated, and Wnt/β-catenin signaling is activated[53]. Growth factor independence 1 (GFI1) may prevent the proliferation of HSCs, and GFI1 is a downstream target of C/EBPα, which prevents cell proliferation when GFI1 levels are low; however, not all cells will become hematopoietic[54]. The differentiation of HSCs is controlled by a series of transcription factors (GATA-1, PU.1, and C/EBP)[55], and C/EBP is closely related to the UPRmt.
The interaction between SIRT7 and NRF1 is a regulatory branch of the UPRmt and is related to cell energy metabolism and proliferation. The expression of SIRT7 declines during the aging of HSCs, and its downregulation induces mitochondrial protein folding stress and contributes to the dysfunction of HSCs. SIRT7 inactivation reduces quiescence, increases mitochondrial protein folding stress, and compromises the regenerative capacity of HSCs. These findings characterize the deregulation of the UPRmt-mediated metabolic checkpoint as a reversible contributing factor to HSC aging[52]. SIRT7 binds to the promoter of NRF1 target genes and thus represses transc
SIRT3 is important in maintaining metabolic regulation, stem cell regeneration, and neuroprotection[59]. SIRT3 protects HSCs from the oxidative damage associated with stress or aging[60]. SIRT3-mediated mitochondrial homeostasis inhibition leads to increased oxidative stress in elderly HSCs, and upregulation of SIRT3 restores the vigor of elderly HSCs, suggesting that high oxidative stress may lead to stem cell senescence, which is reversible in stem cells[60]. Studies have shown that the plasticity of mitochondrial homeostasis controls the aging of HSCs, and the expression of SIRT3 can rejuvenate aging HSCs[60].
Although it is unclear whether the specific loss of UPRmt regulators affects the maintenance of HSCs, studies have shown the importance of mitochondrial protein homeostasis to stem cell viability[61].
Nevertheless, several studies have shown that c-Jun is involved in the quiescence and self-renewal of HSCs[62].
Hematopoietic cells are precursors of a variety of non-hematopoietic tissues that can be differentiated laterally in a specific environment, such as liver cells. Enhancement of the UPRmt or restoration of CLPP levels not only reduces cellular senescence by preventing oxidative stress but also enhances hepatocyte function to prevent the functional decompensation associated with cirrhosis[63].
Although the roles of the UPRmt and UPRmt marker genes in HSCs have been investigated, especially those involved in proliferation, differentiation, and aging, the mechanism by which the UPRmt affects the proliferation, differentiation, and aging of HSCs needs to be discovered, especially the interaction between the UPRmt and SIRT genes.
Neural stem cells (NSCs) reside in the nervous system and have the potential to differentiate into nerve neurons, astrocytes, and oligodendrocytes to produce a large number of brain cells that can self-renew and produce a large number of brain tissues[64]. Mitochondria are central regulators of the fate of NSCs and are critical to both neurodevelopment and adult neurogenesis[65].
FBW7 is highly expressed in the nervous system and controls neural stem cell differentiation and apoptosis via Notch and c-Jun during embryonic development[66]. The AP1-binding site plays an indispensable role in the UPRmt, and c-Jun, a member of the AP1 family of transcription factors, plays an important role in the regulation of the UPRmt.
In the brain, high levels of ATF5 are found in neuronal stem cells, the number of which needs to be reduced via their differentiation into mature neurons or glial cells[67]. ATF5 heterotopic expression in NSCs induces the expression of several olfactory sensory neuron (OSN)-specific genes. ATF5 is expressed in immature OSNs and promotes their maturation into OSNs[68]. Therefore, ATF5 is important for the differentiation of NSCs. In mammals, ATF5 has considerable homology with ATFS-1 in the bZIP domain. ATF5 knockdown impairs cell proliferation, especially in cells expressing an ornithine transcarbamylase-deficient mutant (ΔOTC)[14]. And ΔOTC causes changes in neurocognitive function. Therefore, the UPRmt is important for the differentiation of NSCs.
Wnt, an important developmental regulator, is involved in mediating the UPRmt in nerve cells and intestinal cells[69]. Moreover, the proliferation/differentiation of NSCs is affected by glucocorticoids because of the functions of intracellular signaling pathways such as Wnt[70]. In IEC-specific mouse models, loss of HSP60 chaperone activated the UPRmt and led to mitochondrial dysfunction. The release of Wnt-related paracrine factors from the affected IECs is controlled by factors involved in stem cell proliferation[71].
The genetic and idiopathic forms of Parkinson's disease (PD) are characterized by the loss of dopamine neurons, and the protein levels of CLPP are selectively reduced in the dopaminergic neurons in the brain of PD patients, as determined by post-mortem examination[72]. CLPP, a marker of the UPRmt, may be a useful therapeutic target for PD.
Furthermore, the roles of Wnt genes and the UPRmt in NSCs are largely unknown and should be studied because the Wnt pathway and the UPRmt play vital roles in nervous system development and diseases.
IECs are the most active metabolic site in the body of mammals. IECs constantly renew themselves throughout the life cycle because the stem cells located in intestinal crypts maintain vigorous proliferation and differentiation abilities. Intestinal stem cells (ISCs), located near the base of crypts, terminally differentiate near the crypt opening and produce a variety of intestinal epithelial cell types[73]. ISCs play important roles in maintaining the structural and functional integrity of the intestinal barrier and repair after injury[74]. Under different stress or diet conditions, the proliferation capacity of ISCs is very important for maintaining intestinal integrity. Mitochondrial dysfunction leads to tissue degradation and aging by affecting the homeostasis of somatic cells[75,76]. Therefore, it is very important to understand the biological characteristics of ISCs.
In an IEC-specific mouse model, the deletion of HSP60 activated the UPRmt, resulting in mitochondrial dysfunction, stem cell loss, and impaired intestinal epithelial cell proliferation through CHOP-independent signaling pathways[71]. However, overexpression of epithelial-specific CHOP induced cell cycle arrest in the mice, resulting in impaired wound healing and reduced proliferation of IECs[77]. UPRmt signaling is important to the localization of intestinal epithelial stem cells and their differentiation and lineage commitment[78].
PKR integrates the UPRmt in inflammatory bowel disease (IBD). The endoplasmic reticulum (ER) UPR is initiated via eIF2α phosphorylation and AP1 activation[29]. There may be a connection between mitochondria and the ER with respect to the UPR in IBD.
Hence, the role of the UPRmt in ISCs is poorly understood, and the roles of the UPRmt in the proliferation, differentiation, and aging of ISCs need to be further explored.
Periodontal ligament stem cells (PDLSCs) are undifferentiated mesenchymal cells that remain in the periodontal membrane after the development of periodontal tissue[79].
ROS generated by mitochondria are produced as byproducts of normal oxidative metabolism[80]. In mammals, ROS are also invoked as agents important in processes triggered in cells undergoing apoptosis. Increases in the levels of ROS activate the CHOP branch of the UPRmt and increase the levels of CLPP and HSP10[81]. A study found that the JNK/mitochondrial pathway regulates glycation end products, causing damage and inducing the apoptosis of periodontal membrane stem cells. This pathway is activated by excessive ROS as induced by JNK, known as a stress-activated protein kinase[82]. The phosphorylation activation of JNK induces a decrease in mitochondrial membrane potential, which changes the permeability of the mitochondrial membrane and causes the small-molecule solutes in the cytoplasm to flood into the mitochondrial matrix, resulting in mitochondrial swelling and rupture, triggering the mitochondria-mediated endogenous cell apoptosis pathway, regulating the expression of Bax and Bcl-2, and inducing the apoptosis of PDLSCs. The JNK signaling pathway can activate the proapoptotic protein Bax, inhibit the activity of the antiapoptotic protein Bcl-2, activate c-Jun/AP1 to upregulate proapoptotic proteins, and activate P53 family proteins, thus inducing apoptosis of different stem cells[83,84].
Therefore, many more roles for mitochondria and the UPRmt have been discovered in PDLSCs and are interesting and worthy of further exploration.
Cancer stem cells (CSCs) represent a highly tumorigenic subset of cells in primary tumors[85]. They play important roles in tumorigenesis and tumor progression and recurrence[86]. Mitochondrial changes in CSCs, including morphological changes, abnormal activation of signaling pathways, dysfunction, production of ROS and mitochondrial autophagy, and the UPRmt, are key to the regulation of CSC proliferation and apoptosis and are also among the reasons for the failure of tumor treatment[87]. Therefore, targeting CSCs is crucial for the effective treatment of cancer[88] and finding an attractive target for the development of therapeutics for CSCs.
c-Myc is an important transcriptional regulator in cancer, somatic cell reprog
In addition, the UPRmt is thought to improve the survival rate of cancer cells and thus promote tumor growth[93,94]. ATF5 is highly expressed in undifferentiated NSCs and in a variety of human cancers, including gliomas[95]. Similarities in the expression of ATF5 in rodent, dog, and human tumors and the cross-species efficacy of the CP-d/n ATF5 peptide support the development of an ATF5-targeting approach as a novel and translational therapy for dog gliomas[94]. Analysis of human glioblastoma samples showed that ATF5 expression is negatively correlated with disease prognosis, and interference with ATF5 function can lead to glioma cell death in primary tumors without affecting normal cells surrounding the tumor, indicating that ATF5 is a therapeutic target for glioblastoma[96]. ATF5 may also be a potential therapeutic target for CSC treatments.
HSP70 elimination can lead to depletion of tumor stem cells[97]. Compared with its level in the non-neoplastic prostatic epithelium, HSP60 expression is significantly increased in both early and advanced prostate cancers and in malignant prostate cancer cell lines[98]. The HSP10 pathway is a very active cell signaling network that affects the cell cycle, nuclear and cytoplasmic molecule transport and metabolism, and is an important cause of cancer[99]. HSP10 is highly expressed in a variety of cancers, including lung, pancreatic, and bladder cancers[100]. HSP70, HSP60, and HSP10 play important roles in the UPRmt, maintaining mitochondrial function and quality control; therefore, the modification of HSPs may become a new target for tumor therapy[101].
LONP1 is a UPRmt effector. In mouse models of colorectal cancer and skin cancer, heterozygous LONP1 deficiency attenuated tumor formation[102]. Additionally, in human specimens, elevated LONP1 was associated with a poor cancer prognosis[103].
Mitochondrial CLPP is overexpressed in human cancer cells, which promotes metastasis, and inhibiting CLPP may bring hope for cancer treatment[104].
As a downstream target of the EPHA2 receptor in NSCLCs and in conjunction with EPHA2 in tumor stem cell-like cells, the JNK/C-Jun pathway provides an opportunity for CSC-targeted therapy[105].
Mitochondrial redox homeostasis plays a key role in many biological processes, including biosynthesis and apoptosis, and is therefore a potential target for cancer therapy[106].
Thapsigargin (TG) limits the accumulation of CSCs. The cytoskeleton is rearranged in the presence of TG, and cytoskeleton rearrangement is related to the regulation of the cytoplasm and the UPRmt[107], thus the UPRmt has the potential to treat cancer.
Importantly, discovering the roles and pathways of the UPRmt in CSCs will be very significant for cancer prevention and treatment; thus, the UPRmt might be a novel drug target for cancer treatment.
The UPRmt induces many stem cells, such as induced pluripotent stem cells, mesenchymal stem cells (MSCs), muscle stem cells, and skeletal muscle stem cells.
Compared with somatic cells, induced pluripotent stem cells have fewer mitochondria and undergo less oxidative phosphorylation[31]. There is increasing evidence that during somatic reprogramming, mitochondrial mass is significantly reduced and energy metabolism is switched from oxidative phosphorylation to glycolysis, but the exact molecular mechanisms for these changes remain unclear[108]. They are most likely related to the UPRmt.
For mature MSCs, reducing the level of NAMPT led to a decrease in the intracellular NAD+ concentration, thereby downregulating the expression of SIRT1 after exposure to the NAMPT inhibitor FK866. Young MSCs were induced to become senescent cells. This was mainly caused by the depletion of NAD+ and reduction in SIRT1 activity. NAMPT overexpression can delay the senescence of MSCs during aging[109]. The UPRmt was activated in primary mouse hepatocytes with increased or absent SIRT1 expression[110]. Increasing NAMPT requires a complete mitochondrial NAD salvage pathway and UPRmt-associated protein deacetylase SIRT3[111].
Improving the level of NAD+ cells in mice not only enhances the function of mitochondria but also induces the expression of UPRmt-related genes and inhibition of proteins, thereby preventing skeletal muscle stem cells from aging and prolonging the life of treated mice[112]. In addition to skeletal muscle stem cells, increasing NAD+ can also delay the aging of pigment stem cells[112]. Gastrocnemius muscle differentiated from muscle cells showed low expression of the UPRmt marker CLPP during aging[113]. The UPRmt can delay aging. Additionally, at the cellular level, reduced CLPP impairs myoblast differentiation and cell proliferation and increases eukaryotic initiation factor 2α phosphorylation, thus inhibiting translation[114].
SIRT7 knockout enhanced osteogenic differentiation of bone marrow MSCs[115]. In addition, miR-152 can promote the aging of human dental pulp stem cells by targeting SIRT7 expression[116].
The UPRmt plays a key role in modulating corals' ability to adapt to a changing world, including the production of HSPs and antioxidants[117]. The same may be true of human evolution. Recent studies have found that the UPRmt of C. elegans is very similar to that of mammals[118]. The UPRmt transcription factor ATFS-1 has been shown to regulate HSP70 and other mitochondrial chaperons[10]. ATF5, a homologous gene of ATFS-1, may also regulate HSPs. UBL-5 is a highly conserved protein and is abundant in mitochondria-rich human tissues such as the heart, skeletal muscle, liver, and kidneys[119]. Two UPRmt reporter genes (HSP60 and HSP70) were attenuated by inactivation ofUBL-5 gene encoding C. elegans and animal ubiquitin like small protein[120]. The HSP pathway of the UPRmt may be related to UBL-5. The UPRmt was discovered by the modulation of nuclear genes encoding mitochondrial chaperone proteins by perturbations of the folding environment in mitochondria[121]. In mammalian cells, truncated folding defects of OTC upregulate mitochondrial chaperone proteins HSP60/10, HSP40, and the protease CLPP[122]. This signal transduction pathway may involve the transduction of mitochondrial matrix UPRmt pathway into the nucleus. mtHSP90 inhibitors can induce the UPRmt rapidly[123].
The sirtuin family is critical to the mitochondrial stress response;in particular, SIRT1, SIRT3, and SIRT7 are involved in the UPRmt on different axes[124]. In addition to SIRT3 described above, SIRT7 is involved in the UPRmt. Nicotinamide riboside prevents and reverses non-alcoholic fatty liver disease by inducing the SIRT1 and SIRT3 dependent UPRmt, triggering an adaptive mitotic pathway to increase liver β-oxidation and mitochondrial complex content and activity[125]. There are seven sirtuins in mammals: Sirt1, Sirt2, Sirt6, and Sirt7 are located in the nucleus; Sirt1 and Sirt2 in the cytoplasm; and Sirt3, Sirt4, and Sirt5 in the mitochondrion[126]. This phenomenon may involve the transduction of the mitochondrial UPRmt pathway into the nucleus.
The UPRmt is a double-edged sword with dual effects. The UPRmt initiated by short-term and mild mitochondrial stress, as an intracellular defensive response system, can resist mitochondrial damage and maintain and promote the function of mitochondria. Prolonged and repeated mitochondrial stress may aggravate the irreversible damage to cells by mediating apoptosis[127]. Therefore, controlling the UPRmt effectively is a current challenge, and the role of the UPRmt in stem cells is still unclear and deserves further attention.
The role of the UPRmt in longevity has primarily been examined in C. elegans, an organism that lacks somatic stem cells[128]. Recent reports have shown that activation of the UPRmt, through the administration of an NAD-increasing compound, can rejuvenate stem cells and extend the lifespan of mice[129].It remains to be seen whether the longevity of human stem cells is similar to that of C. elegans and mice.
Despite recent reports of the potential existence of stem cells that might be used to restore the primordial follicle and thereby the oocyte pool, therapeutic interventions during female reproductive aging currently remain limited[130]. The UPRmt has been used to find ways to prolong female reproduction. Study into the relationship between stem cells and the UPRmt in the field of regenerative medicine is ongoing.
Mitochondria are key factors of environmental stability in the body. During this homeostasis, the regulation of mitochondria in stem cells becomes increasingly important[131]. In addition, the UPRmt is inextricably linked to mitochondrial homeostasis.
However, the self-renewal, differentiation, aging, and apoptosis of stem cells are dependent on cell type, and some of the mechanisms need to be further investigated.
Manuscript source: Invited manuscript
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S, Tantau AI S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Li JH
| 1. | Sherratt HS. Mitochondria: structure and function. Rev Neurol (Paris). 1991;147:417-430. [PubMed] [Cited in This Article: ] |
| 2. | Dyall SD, Johnson PJ. Origins of hydrogenosomes and mitochondria: evolution and organelle biogenesis. Curr Opin Microbiol. 2000;3:404-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Searcy DG. Metabolic integration during the evolutionary origin of mitochondria. Cell Res. 2003;13:229-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Durand F, Hoogenraad N. Assessing Mitochondrial Unfolded Protein Response in Mammalian Cells. Methods Mol Biol. 2017;1567:363-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Andreux PA, Houtkooper RH, Auwerx J. Pharmacological approaches to restore mitochondrial function. Nat Rev Drug Discov. 2013;12:465-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt). Mol Cell. 2015;58:123-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 286] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 7. | Jovaisaite V, Mouchiroud L, Auwerx J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J Exp Biol. 2014;217:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 8. | Jovaisaite V, AuwerxJ. The mitochondrial unfolded protein response—synchronizing genomes. Curr Opin Cell Biol. 2015;33:74-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell. 2007;13:467-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 10. | Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337:587-590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 666] [Cited by in F6Publishing: 690] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 11. | Papa L, Germain D. Estrogen receptor mediates a distinct mitochondrial unfolded protein response. J Cell Sci. 2011;124:1396-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Horibe T, Hoogenraad NJ. The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLoS One. 2007;2:e835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411-4419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 709] [Cited by in F6Publishing: 725] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 14. | Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr Biol. 2016;26:2037-2043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 385] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 15. | Tan K, Fujimoto M, Takii R, Takaki E, Hayashida N, Nakai A. Mitochondrial SSBP1 protects cells from proteotoxic stresses by potentiating stress-induced HSF1 transcriptional activity. Nat Commun. 2015;6:6580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol. 2014;34:699-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 17. | Haynes CM, Fiorese CJ, Lin YF. Evaluating and responding to mitochondrial dysfunction: the mitochondrial unfolded-protein response and beyond. Trends Cell Biol. 2013;23:311-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Wardelmann K, Blümel S, Rath M, Alfine E, Chudoba C, Schell M, Cai W, Hauffe R, Warnke K, Flore T, Ritter K, Weiß J, Kahn CR, Kleinridders A. Insulin action in the brain regulates mitochondrial stress responses and reduces diet-induced weight gain. Mol Metab. 2019;21:68-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Voos W, Jaworek W, Wilkening A, Bruderek M. Protein quality control at the mitochondrion. Essays Biochem. 2016;60:213-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Wang X. Stem cells in tissues, organoids, and cancers. Cell Mol Life Sci. 2019;76:4043-4070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Peña S, Sherman T, Brookes PS, Nehrke K. The Mitochondrial Unfolded Protein Response Protects against Anoxia in Caenorhabditis elegans. PLoS One. 2016;11:e0159989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Jeon K, Park K, Jetten AM. Efficient Neural Differentiation using Single-Cell Culture of Human Embryonic Stem Cells. J Vis Exp. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Wanet A, Arnould T, Najimi M, Renard P. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells Dev. 2015;24:1957-1971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 220] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 24. | Heo HJ, Kim HK, Youm JB, Cho SW, Song IS, Lee SY, Ko TH, Kim N, Ko KS, Rhee BD, Han J. Mitochondrial pyruvate dehydrogenase phosphatase 1 regulates the early differentiation of cardiomyocytes from mouse embryonic stem cells. Exp Mol Med. 2016;48:e254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells. 2011;29:486-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 26. | Chen YH, Su CC, Deng W, Lock LF, Donovan PJ, Kayala MA, Baldi P, Lee HC, Chen Y, Wang PH. Mitochondrial Akt Signaling Modulated Reprogramming of Somatic Cells. Sci Rep. 2019;9:9919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | von Elsner L, Hagemann S, Just I, Rohrbeck A. C3 exoenzyme impairs cell proliferation and apoptosis by altering the activity of transcription factors. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:1021-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Chen CW, Liu CS, Chiu IM, Shen SC, Pan HC, Lee KH, Lin SZ, Su HL. The signals of FGFs on the neurogenesis of embryonic stem cells. J Biomed Sci. 2010;17:33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Rath E, Berger E, Messlik A, Nunes T, Liu B, Kim SC, Hoogenraad N, Sans M, Sartor RB, Haller D. Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation. Gut. 2012;61:1269-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 752] [Cited by in F6Publishing: 751] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 31. | Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 467] [Cited by in F6Publishing: 475] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 32. | Gupta S, Knowlton AA. HSP60, Bax, apoptosis and the heart. J Cell Mol Med. 2005;9:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Shan YX, Liu TJ, Su HF, Samsamshariat A, Mestril R, Wang PH. Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells. J Mol Cell Cardiol. 2003;35:1135-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Chan WH. Citrinin induces apoptosis in mouse embryonic stem cells. IUBMB Life. 2008;60:171-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Maiese K. SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015;7:235-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Christensen JH, Nielsen MN, Hansen J, Füchtbauer A, Füchtbauer EM, West M, Corydon TJ, Gregersen N, Bross P. Inactivation of the hereditary spastic paraplegia-associated Hspd1 gene encoding the Hsp60 chaperone results in early embryonic lethality in mice. Cell Stress Chaperones. 2010;15:851-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Liu K, Zhao Q, Liu P, Cao J, Gong J, Wang C, Wang W, Li X, Sun H, Zhang C, Li Y, Jiang M, Zhu S, Sun Q, Jiao J, Hu B, Zhao X, Li W, Chen Q, Zhou Q, Zhao T. ATG3-dependent autophagy mediates mitochondrial homeostasis in pluripotency acquirement and maintenance. Autophagy. 2016;12:2000-2008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Heer R, Hepburn AC, Williamson SC, Kennedy A, El-Sherif A, Soomro NA, Brown CD, Robson CN. Renal differentiation from adult spermatogonial stem cells. Ren Fail. 2013;35:1387-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, Pham J, Anorve S, Chow YC, Izadyar F. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 40. | Zhang C, Wu J. Production of offspring from a germline stem cell line derived from prepubertal ovaries of germline reporter mice. Mol Hum Reprod. 2016;22:457-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Meinhardt A, Wilhelm B, Seitz J. Expression of mitochondrial marker proteins during spermatogenesis. Hum Reprod Update. 1999;5:108-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Udagawa O, Ishihara T, Maeda M, Matsunaga Y, Tsukamoto S, Kawano N, Miyado K, Shitara H, Yokota S, Nomura M, Mihara K, Mizushima N, Ishihara N. Mitochondrial fission factor Drp1 maintains oocyte quality via dynamic rearrangement of multiple organelles. Curr Biol. 2014;24:2451-2458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 43. | Wang T, Babayev E, Jiang Z, Li G, Zhang M, Esencan E, Horvath T, Seli E. Mitochondrial unfolded protein response gene Clpp is required to maintain ovarian follicular reserve during aging, for oocyte competence, and development of pre-implantation embryos. Aging Cell. 2018;17:e12784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Badura-Stronka M, Wawrocka A, Zawieja K, Silska S, Krawczyński MR. Severe manifestation of Leber's hereditary optic neuropathy due to 11778G>A mtDNA mutation in a female with hypoestrogenism due to Perrault syndrome. Mitochondrion. 2013;13:831-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Gispert S, Parganlija D, Klinkenberg M, Dröse S, Wittig I, Mittelbronn M, Grzmil P, Koob S, Hamann A, Walter M, Büchel F, Adler T, Hrabé de Angelis M, Busch DH, Zell A, Reichert AS, Brandt U, Osiewacz HD, Jendrach M, Auburger G. Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors. Hum Mol Genet. 2013;22:4871-4887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 46. | Chen X, Zhong J, Dong D, Liu G, Yang P. Endoplasmic Reticulum Stress-Induced CHOP Inhibits PGC-1α and Causes Mitochondrial Dysfunction in Diabetic Embryopathy. ToxicolSci. 2017;158:275-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Li MW, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med. 2009;15:159-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 48. | Parker GC, Acsadi G, Brenner CA. Mitochondria: determinants of stem cell fate? Stem Cells Dev. 2009;18:803-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Schieke SM, Ma M, Cao L, McCoy JP Jr, Liu C, Hensel NF, Barrett AJ, Boehm M, Finkel T. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. J Biol Chem. 2008;283:28506-28512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | Mantel C, Messina-Graham S, Broxmeyer HE. Upregulation of nascent mitochondrial biogenesis in mouse hematopoietic stem cells parallels upregulation of CD34 and loss of pluripotency: a potential strategy for reducing oxidative risk in stem cells. Cell Cycle. 2010;9:2008-2017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Mohrin M, Widjaja A, Liu Y, Luo H, Chen D. The mitochondrial unfolded protein response is activated upon hematopoietic stem cell exit from quiescence. Aging Cell. 2018;17:e12756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 52. | Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, Haynes CM, Chen D. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347:1374-1377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 350] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 53. | Hsu YC, Wu YT, Tsai CL, Wei YH. Current understanding and future perspectives of the roles of sirtuins in the reprogramming and differentiation of pluripotent stem cells. Exp Biol Med (Maywood). 2018;243:563-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Ay A, Gong D, Kahveci T. Hierarchical decomposition of dynamically evolving regulatory networks. BMC Bioinformatics. 2015;16:161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Zhang C, Yi W, Li F, Du X, Wang H, Wu P, Peng C, Luo M, Hua W, Wong CC, Lee JJ, Li W, Chen Z, Ying S, Ju Z, Shen H. Eosinophil-derived CCL-6 impairs hematopoietic stem cell homeostasis. Cell Res. 2018;28:323-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Barber MF, Michishita-Kioi E, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL, Chen K, Struhl K, Garcia BA, Gozani O, Li W, Chua KF. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012;487:114-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 420] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 57. | Cole A, Wang Z, Coyaud E, Voisin V, Gronda M, Jitkova Y, Mattson R, Hurren R, Babovic S, Maclean N, Restall I, Wang X, Jeyaraju DV, Sukhai MA, Prabha S, Bashir S, Ramakrishnan A, Leung E, Qia YH, Zhang N, Combes KR, Ketela T, Lin F, Houry WA, Aman A, Al-Awar R, Zheng W, Wienholds E, Xu CJ, Dick J, Wang JC, Moffat J, Minden MD, Eaves CJ, Bader GD, Hao Z, Kornblau SM, Raught B, Schimmer AD. Inhibition of the Mitochondrial Protease ClpP as a Therapeutic Strategy for Human Acute Myeloid Leukemia. Cancer Cell. 2015;27:864-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 58. | Tong Z, Wang M, Wang Y, Kim DD, Grenier JK, Cao J, Sadhukhan S, Hao Q, Lin H. SIRT7 Is an RNA-Activated Protein Lysine Deacylase. ACS Chem Biol. 2017;12:300-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 59. | Ghosh S, Zhou Z. SIRTain regulators of premature senescence and accelerated aging. Protein Cell. 2015;6:322-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3:319-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 299] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 61. | Qureshi MA, Haynes CM, Pellegrino MW. The mitochondrial unfolded protein response: Signaling from the powerhouse. J Biol Chem. 2017;292:13500-13506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 62. | Thompson BJ, Jankovic V, Gao J, Buonamici S, Vest A, Lee JM, Zavadil J, Nimer SD, Aifantis I. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J Exp Med. 2008;205:1395-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 63. | Sen B, Rastogi A, Nath R, Shasthry SM, Pamecha V, Pandey S, Gupta KJ, Sarin SK, Trehanpati N, Ramakrishna G. Senescent Hepatocytes in Decompensated Liver Show Reduced UPRMT and Its Key Player, CLPP, Attenuates Senescence In Vitro. Cell MolGastroenterolHepatol. 2019;8:73-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Lecca D, Fumagalli M, Ceruti S, Abbracchio MP. Intertwining extracellular nucleotides and their receptors with Ca2+ in determining adult neural stem cell survival, proliferation and final fate. Philos Trans R SocLond B BiolSci. 2016;371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Watanabe Y, Nakajima K, Ito Y, Akahori Y, Saito F, Woo GH, Yoshida T, Shibutani M. Twenty-eight-day repeated oral doses of sodium valproic acid increases neural stem cells and suppresses differentiation of granule cell lineages in adult hippocampal neurogenesis of postpubertal rats. Toxicol Lett. 2019;312:195-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Jandke A, Da Costa C, Sancho R, Nye E, Spencer-Dene B, Behrens A. The F-box protein Fbw7 is required for cerebellar development. Dev Biol. 2011;358:201-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Hernández IH, Torres-Peraza J, Santos-Galindo M, Ramos-Morón E, Fernández-Fernández MR, Pérez-Álvarez MJ, Miranda-Vizuete A, Lucas JJ. The neuroprotective transcription factor ATF5 is decreased and sequestered into polyglutamine inclusions in Huntington's disease. Acta Neuropathol. 2017;134:839-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Wang SZ, Ou J, Zhu LJ, Green MR. Transcription factor ATF5 is required for terminal differentiation and survival of olfactory sensory neurons. Proc Natl AcadSci USA. 2012;109:18589-18594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Zhang Q, Wu X, Chen P, Liu L, Xin N, Tian Y, Dillin A. The Mitochondrial Unfolded Protein Response Is Mediated Cell-Non-autonomously by Retromer-Dependent Wnt Signaling. Cell. 2018;174:870-883.e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 70. | Odaka H, Adachi N, Numakawa T. Impact of glucocorticoid on neurogenesis. Neural Regen Res. 2017;12:1028-1035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 71. | Berger E, Rath E, Yuan D, Waldschmitt N, Khaloian S, Allgäuer M, Staszewski O, Lobner EM, Schöttl T, Giesbertz P, Coleman OI, Prinz M, Weber A, Gerhard M, Klingenspor M, Janssen KP, Heikenwalder M, Haller D. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat Commun. 2016;7:13171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 72. | Hu D, Sun X, Liao X, Zhang X, Zarabi S, Schimmer A, Hong Y, Ford C, Luo Y, Qi X. Alpha-synuclein suppresses mitochondrial protease ClpP to trigger mitochondrial oxidative damage and neurotoxicity. Acta Neuropathol. 2019;137:939-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Goodell MA, Nguyen H, Shroyer N. Somatic stem cell heterogeneity: diversity in the blood, skin and intestinal stem cell compartments. Nat Rev Mol Cell Biol. 2015;16:299-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 74. | Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 75. | Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 76. | Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl AcadSci USA. 2012;109:21528-21533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 77. | Horndasch M, Lienkamp S, Springer E, Schmitt A, Pavenstädt H, Walz G, Gloy J. The C/EBP homologous protein CHOP (GADD153) is an inhibitor of Wnt/TCF signals. Oncogene. 2006;25:3397-3407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Rath E, Moschetta A, Haller D. Mitochondrial function - gatekeeper of intestinal epithelial cell homeostasis. Nat Rev Gastroenterol Hepatol. 2018;15:497-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 79. | Volponi AA, Pang Y, Sharpe PT. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20:715-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 80. | Raha S, Robinson BH. Mitochondria, oxygen free radicals, and apoptosis. Am J Med Genet. 2001;106:62-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 81. | Oliveira AN, Hood DA. Effect of Tim23 knockdown in vivo on mitochondrial protein import and retrograde signaling to the UPRmt in muscle. Am J Physiol Cell Physiol. 2018;315:C516-C526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Guo J, Weng J, Rong Q, Zhang X, Zhu S, Huang D, Li X, Chen S. Investigation of multipotent postnatal stem cells from human maxillary sinus membrane. Sci Rep. 2015;5:11660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Gupta R, Ghosh S. Putative roles of mitochondrial Voltage-Dependent Anion Channel, Bcl-2 family proteins and c-Jun N-terminal Kinases in ischemic stroke associated apoptosis. Biochim Open. 2017;4:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Schroeter H, Boyd CS, Ahmed R, Spencer JP, Duncan RF, Rice-Evans C, Cadenas E. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem J. 2003;372:359-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 85. | Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2551] [Cited by in F6Publishing: 2608] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 86. | Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1234] [Cited by in F6Publishing: 1578] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 87. | Du FY, Zhou QF, Sun WJ, Chen GL. Targeting cancer stem cells in drug discovery: Current state and future perspectives. World J Stem Cells. 2019;11:398-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 88. | Dhawan P, Ahmad R, Srivastava AS, Singh AB. Cancer stem cells and colorectal cancer: an overview. Curr Top Med Chem. 2011;11:1592-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, Cantor AB, Orkin SH. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143:313-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 529] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 90. | Hasson SA, Kane LA, Yamano K, Huang CH, Sliter DA, Buehler E, Wang C, Heman-Ackah SM, Hessa T, Guha R, Martin SE, Youle RJ. High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature. 2013;504:291-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 91. | Wang W, Nag S, Zhang X, Wang MH, Wang H, Zhou J, Zhang R. Ribosomal proteins and human diseases: pathogenesis, molecular mechanisms, and therapeutic implications. Med Res Rev. 2015;35:225-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 92. | Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, Mayes PA, Wise DR, Thompson CB, Maris JM, Hogarty MD, Simon MC. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22:631-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 93. | Ghosh JC, Siegelin MD, Dohi T, Altieri DC. Heat shock protein 60 regulation of the mitochondrial permeability transition pore in tumor cells. Cancer Res. 2010;70:8988-8993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 94. | Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1844] [Cited by in F6Publishing: 1842] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 95. | York D, Sproul CD, Chikere N, Dickinson PJ, Angelastro JM. Expression and targeting of transcription factor ATF5 in dog gliomas. Vet Comp Oncol. 2018;16:102-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Sheng Z, Li L, Zhu LJ, Smith TW, Demers A, Ross AH, Moser RP, Green MR. A genome-wide RNA interference screen reveals an essential CREB3L2-ATF5-MCL1 survival pathway in malignant glioma with therapeutic implications. Nat Med. 2010;16:671-677. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 97. | Sherman MY, Gabai VL. Hsp70 in cancer: back to the future. Oncogene. 2015;34:4153-4161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 98. | Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099-7105. [PubMed] [Cited in This Article: ] |
| 99. | Czarnecka AM, Campanella C, Zummo G, Cappello F. Heat shock protein 10 and signal transduction: a "capsulaeburnea" of carcinogenesis? Cell Stress Chaperones. 2006;11:287-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Wang K, Li L, Fu L, Yuan Y, Dai H, Zhu T, Zhou Y, Yuan F. Integrated Bioinformatics Analysis the Function of RNA Binding Proteins (RBPs) and Their Prognostic Value in Breast Cancer. Front Pharmacol. 2019;10:140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 101. | Bhat TA, Kumar S, Chaudhary AK, Yadav N, Chandra D. Restoration of mitochondria function as a target for cancer therapy. Drug Discov Today. 2015;20:635-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 102. | Quirós PM, Langer T, López-OtínC. New roles for mitochondrial proteases in health, ageing and disease. Nat Rev Mol Cell Biol. 2015;16:345-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 103. | Quirós PM, Español Y, Acín-Pérez R, Rodríguez F, Bárcena C, Watanabe K, Calvo E, Loureiro M, Fernández-García MS, Fueyo A, Vázquez J, Enríquez JA, López-Otín C. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep. 2014;8:542-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 104. | Seo JH, Rivadeneira DB, Caino MC, Chae YC, Speicher DW, Tang HY, Vaira V, Bosari S, Palleschi A, Rampini P, Kossenkov AV, Languino LR, Altieri DC. The Mitochondrial Unfoldase-Peptidase Complex ClpXP Controls Bioenergetics Stress and Metastasis. PLoSBiol. 2016;14:e1002507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 105. | Song W, Ma Y, Wang J, Brantley-Sieders D, Chen J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 2014;74:2444-2454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 106. | Gong N, Ma X, Ye X, Zhou Q, Chen X, Tan X, Yao S, Huo S, Zhang T, Chen S, Teng X, Hu X, Yu J, Gan Y, Jiang H, Li J, Liang XJ. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat Nanotechnol. 2019;14:379-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 330] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 107. | Kumar S, Chaudhary AK, Kumar R, O'Malley J, Dubrovska A, Wang X, Yadav N, Goodrich DW, Chandra D. Combination therapy induces unfolded protein response and cytoskeletal rearrangement leading to mitochondrial apoptosis in prostate cancer. MolOncol. 2016;10:949-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Liu W, Long Q, Chen K, Li S, Xiang G, Chen S, Liu X, Li Y, Yang L, Dong D, Jiang C, Feng Z, Qin D. Mitochondrial metabolism transition cooperates with nuclear reprogramming during induced pluripotent stem cell generation. BiochemBiophys Res Commun. 2013;431:767-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 109. | Ma C, Pi C, Yang Y, Lin L, Shi Y, Li Y, He X. Nampt Expression Decreases Age-Related Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Sirt1. PLoS One. 2017;12:e0170930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 110. | Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 773] [Cited by in F6Publishing: 833] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 111. | Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095-1107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 765] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 112. | Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J. NAD⁺ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436-1443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 708] [Cited by in F6Publishing: 776] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 113. | Cordeiro AV, Brícola RS, Braga RR, Lenhare L, Silva VRR, Anaruma CP, Katashima CK, Crisol BM, Simabuco FM, Silva ASR, Cintra DE, Moura LP, Pauli JR, Ropelle ER. Aerobic Exercise Training Induces the Mitonuclear Imbalance and UPRmt in the Skeletal Muscle of Aged Mice. J GerontolABiolSci Med Sci. 2020;75:2258-2261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 114. | Deepa SS, Bhaskaran S, Ranjit R, Qaisar R, Nair BC, Liu Y, Walsh ME, Fok WC, Van Remmen H. Down-regulation of the mitochondrial matrix peptidase ClpP in muscle cells causes mitochondrial dysfunction and decreases cell proliferation. Free Radic Biol Med. 2016;91:281-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 115. | Yang S, Guo S, Tong S, Sun X. Promoting Osteogenic Differentiation of Human Adipose-Derived Stem Cells by Altering the Expression of Exosomal miRNA. Stem Cells Int. 2019;2019:1351860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 116. | Wang J, Zhu MC, Kalionis B, Wu JZ, Wang LL, Ge HY, Chen CC, Tang XD, Song YL, He H, Xia SJ. Characteristics of circular RNA expression in lung tissues from mice with hypoxiainduced pulmonary hypertension. Int J Mol Med. 2018;42:1353-1366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 117. | Dimos BA, Mahmud SA, Fuess LE, Mydlarz LD, Pellegrino MW. Uncovering a mitochondrial unfolded protein response in corals and its role in adapting to a changing world. Proc BiolSci. 2019;286:20190470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell. 2010;37:529-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 380] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 119. | Friedman JS, Koop BF, Raymond V, Walter MA. Isolation of a ubiquitin-like (UBL5) gene from a screen identifying highly expressed and conserved iris genes. Genomics. 2001;71:252-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics. 2006;174:229-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 121. | Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci. 2004;117:4055-4066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 122. | Kirstein-Miles J, Morimoto RI. Caenorhabditis elegans as a model system to study intercompartmentalproteostasis: Interrelation of mitochondrial function, longevity, and neurodegenerative diseases. Dev Dyn. 2010;239:1529-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 123. | Kang BH, Plescia J, Song HY, Meli M, Colombo G, Beebe K, Scroggins B, Neckers L, Altieri DC. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J Clin Invest. 2009;119:454-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 124. | Weng H, Ma Y, Chen L, Cai G, Chen Z, Zhang S, Ye Q. A New Vision of Mitochondrial Unfolded Protein Response to the Sirtuin Family. CurrNeuropharmacol. 2020;18:613-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 125. | Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V, Kim B, Park YK, Piersigilli A, Pham TX, Yang Y, Ku CS, Koo SI, Fomitchova A, Cantó C, Schoonjans K, Sauve AA, Lee JY, Auwerx J. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190-1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 126. | Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion. 2010;10:12-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 127. | Tian Y, Merkwirth C, Dillin A. Mitochondrial UPR: A Double-Edged Sword. Trends Cell Biol. 2016;26:563-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 128. | Melber A, Haynes CM. UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28:281-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 292] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 129. | Wu LE, Sinclair DA. Restoring stem cells - all you need is NAD. Cell Res. 2016;26:971-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 130. | Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19:67-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 131. | Zheng CX, Sui BD, Qiu XY, Hu CH, Jin Y. Mitochondrial Regulation of Stem Cells in Bone Homeostasis. Trends Mol Med. 2020;26:89-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 132. | Anderson AJ, Jackson TD, Stroud DA, Stojanovski D. Mitochondria-hubs for regulating cellular biochemistry: emerging concepts and networks. Open Biol. 2019;9:190126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 133. | Donovan EL, Lopes EBP, Batushansky A, Kinter M, Griffin TM. Independent effects of dietary fat and sucrose content on chondrocyte metabolism and osteoarthritis pathology in mice. Dis Model Mech. 2018;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 134. | Gan Z, Fu T, Kelly DP, Vega RB. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 2018;28:969-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 135. | Lelegren M, Liu Y, Ross C, Tardif S, Salmon AB. Pharmaceutical inhibition of mTOR in the common marmoset: effect of rapamycin on regulators of proteostasis in a non-human primate. Pathobiol Aging Age Relat Dis. 2016;6:31793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 136. | Wang Y, Li Y, Song L, Jiang S, Zhang S. The transplantation of Akt-overexpressing amniotic fluid-derived mesenchymal stem cells protects the heart against ischemia-reperfusion injury in rabbits. Mol Med Rep. 2016;14:234-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 137. | Yao YL, Yang WM. Beyond histone and deacetylase: an overview of cytoplasmic histone deacetylases and their nonhistone substrates. J Biomed Biotechnol. 2011;2011:146493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |