Published online Jul 26, 2021. doi: 10.4252/wjsc.v13.i7.914
Peer-review started: March 13, 2021
First decision: April 6, 2021
Revised: April 10, 2021
Accepted: July 12, 2021
Article in press: July 12, 2021
Published online: July 26, 2021
Kidney diseases are a prevalent health problem around the world. Multidrug therapy used in the current routine treatment for kidney diseases can only delay disease progression. None of these drugs or treatments can reverse the progression to an end-stage of the disease. Therefore, it is crucial to explore novel therapeutics to improve patients’ quality of life and possibly cure, reverse, or alleviate the kidney disease. Stem cells have promising potentials as a form of regenerative medicine for kidney diseases due to their unlimited replication and their ability to differentiate into kidney cells in vitro. Mounting evidences from the administration of stem cells in an experimental kidney disease model suggested that stem cell-based therapy has therapeutic or renoprotective effects to attenuate kidney damage while improving the function and structure of both glomerular and tubular compartments. This review summarises the current stem cell-based therapeutic approaches to treat kidney diseases, including the various cell sources, animal models or in vitro studies. The challenges of progressing from proof-of-principle in the laboratory to widespread clinical application and the human clinical trial outcomes reported to date are also highlighted. The success of cell-based therapy could widen the scope of regenerative medicine in the future.
Core Tip: Stem cells have the potential to be the next regenerative medicine to treat kidney diseases. There is mounting evidence suggesting that stem cell-based therapy has renoprotective effects to attenuate kidney damage while improving kidney function. This review summarises the current stem cell-based therapy approaches to treat kidney diseases in experimental models and the outcomes from human clinical trials reported to date.
- Citation: Wong CY. Current advances of stem cell-based therapy for kidney diseases. World J Stem Cells 2021; 13(7): 914-933
- URL: https://www.wjgnet.com/1948-0210/full/v13/i7/914.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i7.914
Kidney disease is a prevalent global health problem. A new analysis suggested that the global prevalence of chronic kidney disease (CKD) in the year 2017, was 9.1% (697.5 million cases)[1,2]. The World Health Organization has estimated that as many as 5 to 10 million people die annually from kidney diseases worldwide[3]. By 2040, CKD is projected to be the fifth leading cause of death worldwide[4].
To date, there has been no significant breakthrough in the medical treatment of kidney diseases, whereas the current routine treatment consisting of multidrug therapies can only delay the disease’s progression. These drugs cannot reverse the progression into the end-stage kidney disease (ESKD). The current therapeutic repertoire to prolong the lifespan of ESKD patients is limited to kidney replacement therapy, dialysis or organ transplantation[5]. Due to the high medical cost involved in dialysis therapy, which also compromises the patients’ quality of life, dialysis is not an ideal solution. This is primarily because dialysis does not restore or substitute all kidney functions[6]. Meanwhile, the severe shortage of organ donors and potential organ rejection risks limit the practice of kidney transplantations[7]. Therefore, it is crucial that medical researchers explore novel therapeutics to improve the quality of life for patients with kidney diseases and to potentially cure, reverse, or alleviate the kidney disease.
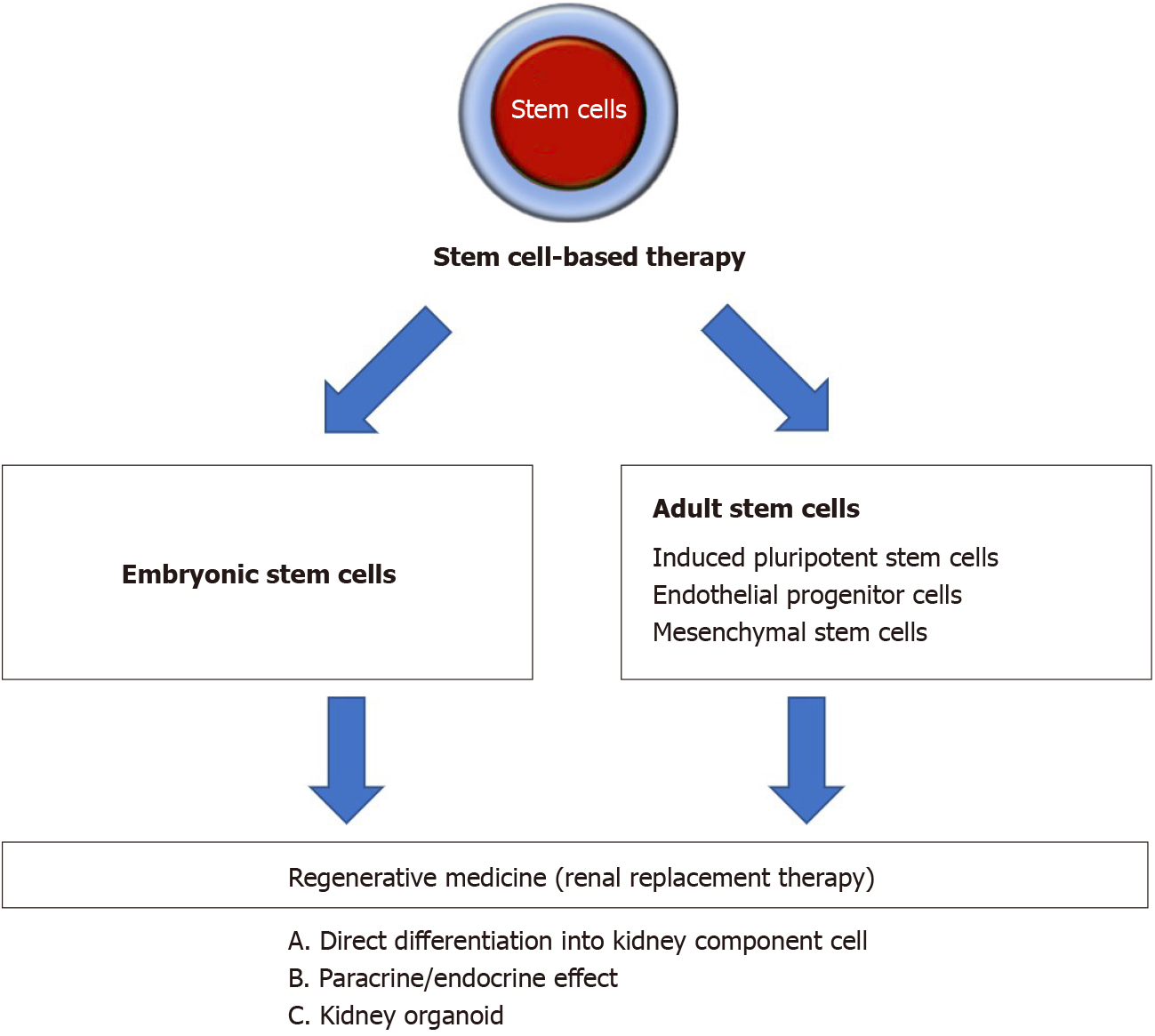
Stem cells are defined as cells capable of self-renewal and can differentiate into a variety of cell types. Moreover, stem cells possess cellular plasticity and easily expand in vitro, which are the beneficial properties of stem cell therapy. Stem cells have been extensively explored in treating cardiac, neuron, vascular, immunological, and kidney diseases[8,9]. In some countries, there are stem cell therapies based on mesenchymal stem cells (MSCs), which are available as commercial products approved by local regulatory agencies for specific diseases or health indications[10]. Thus, this form of intervention can pave the way as the next regenerative medicine for human diseases. Figure 1 showed an overview of stem cell-based strategies to treat kidney disease.
This present article reviews stem cell-based therapy in kidney regeneration based on animal models and in vitro studies, as well as discusses its potential for clinical application and the challenges in translating from animal models to clinical application.
Embryonic stem cells (ESCs) are pluripotent cells with unlimited differentiation potentials. Several research groups have demonstrated that mouse ESCs can integrate into kidney compartments, suggesting the potential value of stem cells in kidney repair. Implantation of ESCs directly into mouse embryonic kidney culture resulted in ESC-derived tubules and proximal tubular cells[11]. Exposing ESCs to specific inducers or factors also caused the induction of cells to differentiate into kidney lineage cells in vitro[12,13]. Kim and Dressler[14] induced mouse ESCs to differentiate into renal progenitor cells and then incorporated these cells' into the tubular epithelial by injection into embryonic kidney cultures. In another study, when intact rat kidneys were decellularised in this manner, the kidney’s intricate architecture was preserved, and the seeded ESCs could proliferate within the glomerular, vascular, and tubular structures[15]. Vazquez-Zapien et al[16] further reported that after mice with cisplatin-induced kidney failure received mouse ESCs via injection, the mortality rate decreased significantly and prevented further disease-related histological deterioration.
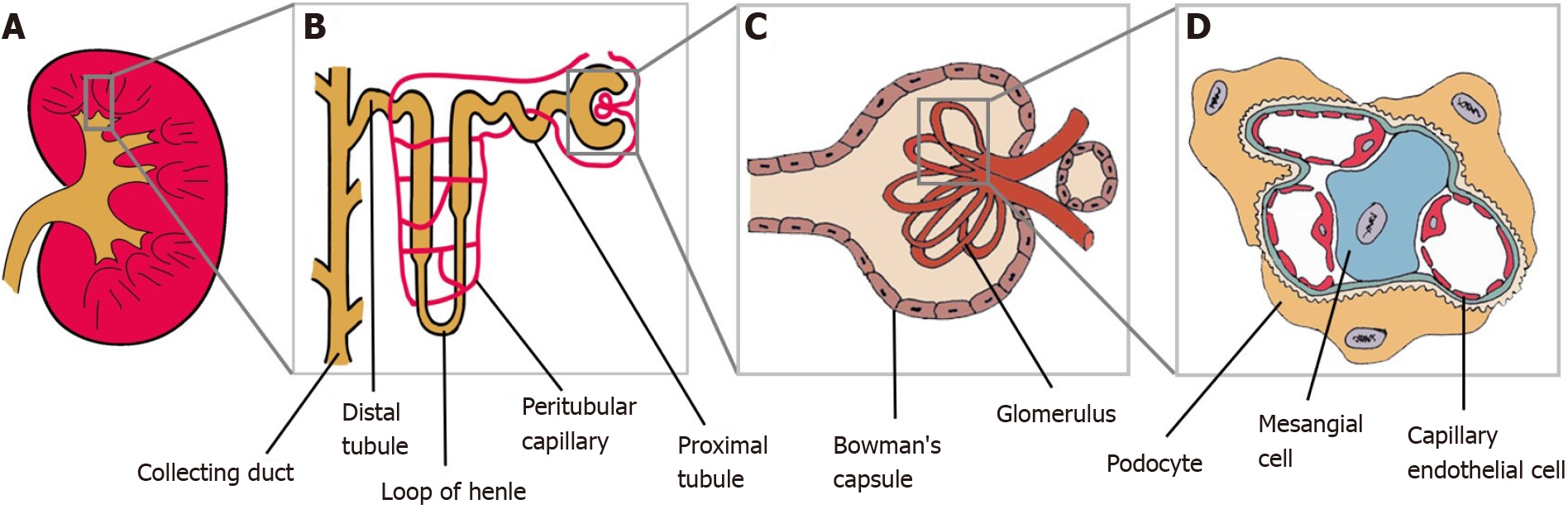
The kidney, on another hand, is a very complex organ made of several cell types (Figure 2). Therefore, it is an intricate organ to reconstruct. Many researchers have worked on a protocol to induce ESCs into generating complex structures to resemble kidneys with multiple renal cell types and capable of self-organization, termed as organoids[17,18]. Tan et al[19] reported that mouse ESCs-derived nephron progenitors, aggregated with primary ureteric bud, formed kidney organoids with full nephron structures.
Despite its clinical potentials, the risk of tumorigenicity, together with legal and ethical concerns, continue to hinder the development of ESC-based therapies. Additionally, ESC-derived differentiated cells are allogeneic in nature and can therefore express specific surface proteins to trigger the recipient’s immune system. Therefore, acute and chronic rejection, or graft vs host disease, can occur due to the use of allografts if they fail to achieve immunocompatibility with the recipient[20,21].
Induced pluripotent stem cells (iPSCs) share many regenerative properties to ESCs. In 2006, Takahashi and Yamanaka showed that mouse adult fibroblasts can be reprogrammed into iPSCs, by introducing four transcription factors (OCT4, SOX2, KLF4 and c-MYC)[22]. This was a breakthrough finding that became a landmark in stem cell research. The development of iPSCs-based therapies could overcome the specific issues related to the use of ESCs, such as ethical concerns due to the cells’ source and the potential of cell rejection by the recipient patient.
The use of ESCs has its controversies due to certain parties who are in the opinion that destroying an embryo for its ESCs is akin to killing an unborn child. Hence, iPSCs are an attractive alternative to ESC-like stem cells as the iPSCs can be generated from adult cells. These cells also retain the genetic background and peculiar epigenetic-memory of their parent cell, thus possibly avoiding any strong immune response. To date, iPSCs have been generated from fibroblasts[23], umbilical cord blood[24], peripheral blood[25,26], and keratinocytes[27]. Researchers have also successfully generated iPSCs from mesangial cells[28], renal tubular cells[29], and renal epithelial cells[30,31]. Reprogrammed iPSCs from kidney cells are believed to potentially aid in the study of genetic kidney diseases, that may lead to the development of novel therapies.
The potential of iPSCs in kidney regeneration have been explored, including establishing unique methods to stimulate human iPSCs to differentiate into kidney lineages[32-34] or three-dimensional structures of the kidney[35]. Toyohara et al[36] had established a multistep differentiation protocol to induce human iPSCs to differentiate into renal progenitors capable of constructing three-dimensional proximal renal tubule-like structures in vitro. The same group subsequently discovered that renal subcapsular transplantation of these human iPSC-derived renal progenitors ameliorated the acute kidney injury (AKI) in the animal model.
In addition to relying on the differentiation ability of iPSCs, researchers have also used the renotropic factors produced by the iPSCs in kidney regeneration. Trans
From recent developments, iPSCs can now be directed to differentiate and generate kidney organoids resembling the human kidney in vitro. An artificially created human kidney can be applied in regenerative medicine, and in developmental, toxicity, and disease models[39-41]. Furthermore, using patients' own iPSCs to generate high-quality kidney organoids enables drug validation in a patient-specific manner. This is contributed by the tight correlation between the patients’ individual genetic background and drug responsiveness[42,43]. Melissa H Little’s group had successfully generated human iPSCs-derived kidney organoids that had all the anticipated kidney cell types. These organoids possessed nephrons segmented into the glomerulus, proximal tubule, loop of Henle, and distal tubule along with the collecting duct, endothelial network, and renal interstitium[44-46]. Meanwhile similarly, the Izpisua-Belmonte’s group had also generated a kidney organoid containing glomeruli with podocytes, proximal and distal tubule cells, and endothelial cells[42]. Morizane et al[47] reported several differentiation protocols for creating kidney organoids with epithelial nephron-like structures. These organoids expressed the markers for podocytes, proximal tubules, loops of Henle, and distal tubules.
Other research groups reported that iPSCs derived from various cell types are not identical in their differentiation capacity[48,49]. This is likely to happen because iPSCs maintain the epigenetic memory of their parental cells[50]. Therefore, renal paren
Endothelial progenitor cells (EPCs) have essential roles in maintaining vascular integrity and in repairing any form of endothelial damage[51]. EPCs can be isolated from different cell sources, mainly from the readily available bone marrow, cord blood, and peripheral blood[52]. The beneficial effects of EPCs-based therapies have been shown in studies performed using different models of kidney diseases such as AKI, CKD, and renal artery stenosis. In an animal model of renal ischemia/ reperfusion (I/R)-induced AKI, renal artery-derived EPC-like cells integrated into the endothelium after AKI, led to decreased levels of serum creatinine (SCr) and albuminuria while blood flow improved[53]. Patschan et al[54] further demonstrated that the systemic injection of peripheral blood-derived early EPCs decreased SCr, ameliorated interstitial fibrosis, and subsequently reduced the progression to CKD after AKI.
The possible effect of EPC treatment on CKD progression was studied in an animal model[55]. In this study, bone marrow-derived EPCs that were homed into the injured kidney, prevented the inflammatory condition from adversely affecting the kidney, and successfully preserved kidney function and structure. Huang et al[56] demonstrated a rodent model injected with peripheral blood-derived EPCs and observed effective inhibition of the propagation of CKD and the deterioration of kidney function. The injected cells enhanced angiogenesis and blood flow, and had anti-oxidative capacity while suppressing inflammation, oxidative stress, apoptosis, and fibrosis[56]. Meanwhile, in renal artery stenosis models, peripheral blood derived-EPCs demonstrated renoprotective effects after injection into the stenotic kidney, by improving microvascular density and kidney functions along with diminishing fibrosis[57-59].
MSCs, or as recently referred to as mesenchymal stromal cells, were first discovered by Friedenstein and his colleagues from bone marrow[60]. Over the years, researchers have found that MSCs can be isolated from various organs or tissues, such as adipose tissue[61], umbilical cord[62,63], placenta[64], peripheral blood[65], amniotic fluid[66], and skeletal muscles[67].
Bone marrow is the most commonly used source for MSCs in clinical treatments, including treating kidney diseases. However, the use of bone marrow derived-MSCs (BM-MSCs) became limited because of a high degree of viral exposure, and that the cell proliferation/differentiation capability significantly decreases as the donor’s age increases[68]. Therefore, researchers began exploring other types of MSCs for kidney regeneration. Among the many sources, adipose tissue-derived MSCs (AD-MSCs) and umbilical cord-derived MSCs (UC-MSCs) have become desirable candidates because a large amount of the MSCs can be obtained using relatively minimal invasive pro
In the field of kidney disease, MSCs are among the most efficient type of cell population for activating regeneration in a damaged kidney[70]. Pre-clinical reports have demonstrated the therapeutic potential of MSCs in animal models of AKI and CKD[71,72]. According to a systematic review of more than 70 articles, MSCs are among the most effective cell populations to treat experimental CKD[73]. Meanwhile, in a meta-analysis involving animal models of chronic and AKI, MSCs led to kidney regeneration despite the variable modes of administration (arterial, venous or renal)[71]. There is evidence suggesting the beneficial effects of MSCs in blocking AKI-CKD transition, a term used to describe an incomplete recovery from AKI resulting in long-term functional deficits, such as CKD[74].
In an experiment performed by Brasile et al[75], when ischemically damaged human kidneys were perfused ex vivo with MSCs for 24 h, kidney regeneration was documented. The MSCs-based treatment caused the kidneys to synthesize significantly lower levels of inflammatory cytokines. Compared to exsanguinous metabolic support perfusion alone, there was a significant increase in the number of renal cells undergoing mitosis in the MSCs-treated kidneys[75].
Numerous studies have demonstrated that MSCs can either differentiate into renal cells in general[76] or specifically into kidney component cells such as renal epithelial cells[77-80], mesangial cells[81,82], and endothelial cells[83,84].
Many studies have demonstrated the efficacy of BM-MSCs in the treatment of kidney disease using animal models of AKI[85,86], podocyte injury[87], and glomerulonephropathy[88,89]. Morigi et al[90,91] is among the first groups to demonstrate the renoprotective role of BM-MSCs and documented the therapeutic potential of human BM-MSCs in the treatment of kidney diseases, leading to survival in animal models. Transplantation of human BM-MSCs into cisplatin-induced AKI mice resulted in markedly improved kidney function and recovery by accelerating tubular proliferation and reducing the number of tubules affected by apoptosis, necrosis and tubular lesions. A similar form of protection was conferred by injected BM-MSCs in a glycerol-induced pigment nephropathy model[77,92] and I/R-induced AKI[86,93]. More importantly, infused BM-MSCs have shown to enhance kidney functional recovery even when administered 24 h after the injury[94]. Furthermore, BM-MSCs were more effective in treating AKI in the animal model compared to candesartan, which is an angiotensin II blocker[95]. In essence, there is good evidence that when MSCs are transplanted in toxic and ischemic animal models, the cells protect the animals against AKI and accelerate the recovery phase.
BM-MSCs have also shown promises in the treatment of CKD in animal models. BM-MSCs prevented the loss of peritubular capillaries and slowed down the progression of proteinuria (protein in the urine)[96]. During the initial phase of the immune response before the onset of CKD, these cells also reduced kidney fibrosis[97]. According to a histological analysis of a rat model with CKD, BM-MSCs reduced glomerulosclerosis, resulting in preservation of kidney function and attenuation of kidney injury[98]. Additionally, when animal models with CKD were treated with BM-MSCs, there were reduced progression of proteinuria and scarce engraftment of these cells in the kidneys. These observations suggested that these beneficial effects were probably caused by cytokines or growth factors, which are also known as the paracrine secretion of mediators[99].
In addition to the ability of BM-MSCs to differentiate into renal cells, more recent reports suggested that BM-MSCs exert protective and regenerative effects on kidneys by their paracrine anti-inflammatory, anti-fibrotic, and vascularisation properties[93,100]. According to reports, BM-MSCs can transfer biological cues via the secretion of extracellular vesicles (EVs) to promote regenerative processes in injured renal cells[101-103].
Several studies investigated the effects of BM-MSCs in experimental models of kidney organ transplantation[104,105]. Most studies focused on the intervention’s efficacy through prolonged graft survival and inhibition of the rejection process[106-108]. Given the advantage of BM-MSCs having low immunogenicity and immunoregulatory properties, BM-MSCs can reduce alloimmune injury and immune suppression-related side effects to optimise preservation of the transplanted kidney’s functions[109,110].
AD-MSCs are highly abundant in adipose tissues and can be easily extracted via liposuction, a method which is widely used in the clinical setting. Adipose tissue may become the preferred source of MSCs due to its less invasive procurement and higher MSCs concentration than those found in the bone marrow[111]. Their allergenic transplantation via the intra-renal route contributed to a low degree of necrosis, but caused higher vascularisation of the renal parenchyma in Wister rats[112]. There are reports on the therapeutic effect of AD-MSCs in AKI-induced animal models. Kim et al[113] have demonstrated that AD-MSCs reduced apoptotic cell death while simultaneously reducing the activation of p53, c-Jun NH2-terminal kinase and extracellular signal-regulated kinase, which are inflammation-related molecules. These effects resulted in increased survival rate of the AKI-induced animals. Katsuno et al[114] further discovered that human AD-MSCs cultured in low serum secreted high levels of hepatocyte and vascular endothelial growth factors. When these cells were transplanted into AKI-induced rats, they enhanced the attenuation of kidney damage[114]. Even though adipose tissue is a good source of MSCs, AD-MSCs have been less effective in proliferative and kidney regenerative activities compared to BM-MSCs[112].
The easy collection of UC-MSCs provides a new abundant source of MSCs. The usage of UC-MSCs, transforms a medical waste into a beneficial product with clinical applications[115]. Compared to MSCs from other sources, UC-MSCs have low immunogenicity, thus preventing the occurrence of immune rejection in allogeneic transplantation[68,116]. Moreover, UC-MSCs have a greater proliferation capacity compared to BM-MSCs and AD-MSCs[117,118]. UC-MSCs also show no sign of senescence over several passages[119], so mass cell production of UC-MSCs is highly possible without causing the loss of cell potency.
More studies are being performed to demonstrate that human MSCs isolated from the umbilical cord exert superior therapeutics effects compared to other sources of MSCs. Researchers also found that when UC-MSCs were implanted into AKI-induced mice, the cells exerted renoprotective effects by inducing tubular cell proliferation[120] and promoting glomerular filtration that prolonged the animals’ survival[121]. Meanwhile, in a CKD-induced rodent model, transplanted UC-MSCs inhibited inflammation and fibrosis while the expression of growth factors was promoted. These effects protected the injured kidney tissues and prevented disease progression[122,123].
In addition to BM-MSCs, AD-MSCs and UC-MSCs, researchers have also used other types of MSCs that are less commonly studied for kidney regeneration studies. The efficacy of cord blood-derived MSCs (CB-MSCs) administration in the restoration of kidney function had been reported in animal AKI models[124]. This study demonstrated that CB-MSCs promoted kidney regeneration and prolonged the survival of the animal. Based on this study, the paracrine action of CB-MSCs on the tubular cells may have been mediated by the reduction of oxidative stress, apoptosis, and inflammation. Hauser et al[125] and George et al[126] found that amniotic fluid-derived MSCs (AF-MSCs) possess the same characterisation as BM-MCSs, facilitating functional and structural improvement in a rat model of CKD. Sedrakyan et al[127] showed the injection of AF-MSCs in mice delays the progression of renal fibrosis. Meanwhile, in a CKD-induced rat model in a study by Cetinkaya et al[128], transplanted placenta-derived MSCs (PL-MSCs) alleviated kidney damage and inhibited fibrosis-induced apoptosis. PL-MSCs were also used to treat kidney injury and inflammation in lupus nephritis (LN) mice[129].
EVs are small membrane vesicles secreted by various cells and found in most body fluids[130]. This review uses the general term, EVs, because there is no method to precisely identify the vesicles[131]. EVs can be classified into three major categories, which are exosomes, microvesicles, and apoptotic bodies. In the current context, researchers suggest that EVs could be transferred to injured cells to restrain tissue injury, reduce inflammation, inhibit apoptosis, and induce cell cycle re-entry of resident cells, all of which lead to cell proliferation, tissue self-repair and regeneration[132]. Upon administration with a therapeutic regimen, EVs will mimic stem cells' effects in various experimental models.
The use of the stem cell-derived EVs could have multiple advantages in clinical application including bypassing most of the safety concerns related to stem cell therapy, such as cellular contamination with oncogenic cells, tumorigenicity, and emboli formation after transplantation[133]. Similarly, EVs also enable a wide range of potential manipulations to carrier molecules for improvements in delivery and desired effects[134]. EVs can also be safely stored in medical facilities without losing any of their functions[135].
Several studies had provided convincing evidence on the regenerative potential of EVs released by stem cells, specifically MSCs, in different models of kidney injury[136,137]. In vitro studies have demonstrated the potential of MSCs-derived EVs to transfer mRNA, miRNA, and proteins to renal cells[132]. Currently, this cell-free therapy is being studied in animal models of AKI[138-140] and CKD[141,142]. Intravenous MSC-derived EVs exert renoprotective effects by reducing renal cell damage and apoptosis while enhancing proliferation of the renal cells. These effects lead to improved kidney function, similar to those induced by MSCs, as reported in rats injected with EVs from EPCs[143,144] and iPSCs[145,146].
When a patient living with ESKD undergoes a kidney transplantation, the patient’s quality of life significantly improves[5]. Nevertheless, chronic allograft nephropathy limits organ survival, eventuating in the patient having to undergo kidney transplantation more than once in a lifespan[147]. The administration of EVs after kidney transplantation was found to ameliorate I/R injury in both the acute and chronic stages, favour tolerance and prolong allograft survival[148].
The preconditioning of a kidney with stem cells-derived EVs may also conveniently limit tissue damage caused by chronic allograft nephropathy. Evidence showed that the MSCs-derived EVs delivered in the perfusate during organ cold perfusion for 4 h protected the kidney from reperfusion damage and can preserve the organ’s enzymatic machinery, which is essential for cell viability[149].
Despite numerous animal experiments to demonstrate the effectiveness of stem cell therapy in kidney disease, outcomes from those animal models were unsuccessfully reproduced in human clinical studies in entirety[150]. The failure to translate the promising results from an animal experiment, which has a sound and standardised design and conduct, to a clinical application happens due to variations in the animal model and the human physiology[151].
Human kidney diseases are typically artificially induced in the animal model. The induced injury is generally acute, unphysiological, and cannot epitomise the human kidney diseases' complex pathological features[152,153]. It is thus difficult to precisely simulate or predict the response of the human kidney disease to treatment by using an animal model. CKD animal experiments are insufficient to reflect the disease’s conditions because other factors such as age, sex, and comorbidities are not re
Both humans and animals may have the same protein functions but there are species-specific differences in the molecular regulation of genes. This causes difficulty in extrapolating an outcome from an animal gene analysis to the physiological conditions in the human body[157]. The differences between the animals' and humans' immune systems is another reason for the failure to translate therapeutics with promising outcome in in vivo studies into a clinical application with potential[158].
Up until March 2021, more than 40 clinical trials, either on-going or completed, involving the use of stem cell-based therapy in the treatment of kidney diseases have been registered in the United States National Library of Medicine (ClinicalTrials.gov). Most of these stem cell clinical trials for kidney disease use MSCs in their approach. Table 1 showed the completed clinical trials of MSC- and EPC-based therapies in kidney diseases.
| Ref. | Condition or disease | Trial registration | Source | Main findings |
| Togel et al[159], Westenfelder et al[160] | Patients with a high risk of developing AKI after undergoing cardiac surgery | NCT00733876 | Allogeneic BM-MSCs | MSC infusion was safe |
| None of the patients developed postoperative AKI or subsequent loss of kidney function | ||||
| Protection against early and late post-surgery kidney function deterioration | ||||
| Reduction in length of hospital stay | ||||
| Swaminathan et al[161] | Patients who experienced AKI 48 hr after cardiac surgery | NCT01602328 | Allogeneic BM-MSCs | No significant difference between groups in 30-d all-cause mortality or dialysis |
| No reduction in the time to recover kidney function | ||||
| No difference in adverse events between groups | ||||
| Makhlough et al[164] | CKD | NCT02195323 | Autologous BM-MSCs | MSC infusion was safe and tolerated. |
| No significant changes in eGFR and SCr | ||||
| Villanueva et al[162] | CKD | NA | Autologous AD-MSCs | MSC infusion was safe and not associated with adverse effects |
| Statistically significant improvement in urinary protein excretion but not in GFR | ||||
| Lee et al[178], Yang et al[179] | CKD at stage III or IV | NA | Autologous CD34+ EPCs | MSC infusion was safe and tolerated |
| No additional benefit from EPCs up to a follow-up period | ||||
| Significantly lower unfavourable clinical outcomes (dialysis or death) in treatment group | ||||
| Makhlough et al[163] | CKD due to autosomal dominant polycystic kidney disease | NCT02166489 | Autologous BM-MSCs | MSC infusion was safe and tolerated |
| No significant changes in eGFR and SCr | ||||
| Packham et al[180] | Diabetic nephropathy (type 2) | NCT01843387 | Allogeneic BM-MSCs | MSC infusion was safe and not associated with acute adverse events |
| Stabilisation and improvement in eGFR and mGFR | ||||
| Saad et al[181] | Atherosclerotic renovascular disease | NCT02266394 | Autologous AD-MSCs | MSC infusion was safe and well tolerated. |
| Increment in cortical perfusion and renal blood flow | ||||
| Reduction in renal tissue hypoxia within poststenotic kidney | ||||
| Sun et al[165], Liang et al[166] | Refractory SLE | NCT00698191 | Allogeneic BM-MSCs | MSC infusion was safe and tolerated |
| Improvement in disease activity | ||||
| Stabilisation in kidney function | ||||
| Sun et al[167] | Refractory SLE | NCT00698191 | Allogeneic UC-MSCs | MSC infusion was safe and tolerated |
| Improvement in disease activity | ||||
| Stabilisation in kidney function | ||||
| Wang et al[168] | Refractory SLE | NCT01741857 | Allogeneic UC-MSCs | MSC infusion was safe. |
| Reduction in proteinuria 24 hr after transplantation, with statistical differences at 9- and 12-mo follow-ups | ||||
| Deng et al[171] | LN (class III or IV) | NCT01539902 | Allogeneic UC-MSCs | No apparent additional effect over and above standard immunosuppression |
| Barbado at al[170] | Active and refractory LN | NA | Allogeneic BM-MSCs or UC-MSCs | Significant improvement in proteinuria levels during the 1st month after treatment |
| The ameliorations were sustained throughout the follow-up period | ||||
| Tan et al[177] | Kidney transplant | NCT00658073 | Autologous BM-MSCs | MSC infusion was not associated with adverse events and did not compromise kidney transplant survival |
| Lower incidence of acute rejection | ||||
| Reduction in risk of opportunistic infection, and better estimated kidney function at 1 yr | ||||
| Reinders et al[182] | Kidney transplant | NCT00734396 | Autologous BM-MSCs | MSC infusion was feasible and safe |
| Increment in incidence of opportunistic infection | ||||
| Perico et al[172,174] | Kidney transplant | NCT00752479 | Autologous BM-MSCs | MSC infusion was safe and feasible |
| Allowed enlargement of Treg in the peripheral blood | ||||
| Controlled memory CD8+ T cell function | ||||
| No major side effects during long-term follow-up | ||||
| Erpicum et al[176] | Kidney transplant | NCT01429038 | Autologous BM-MSCs | MSC infusion was safe |
| Increment in regulatory T cell proportion and with improved early allograft function | ||||
| Perico et al[172-173] | Kidney transplant | NCT02012153 | Autologous BM-MSCs | MSC infusion was safe |
| Reduction in circulating memory CD8+ T cells and donor-specific CD8+ T-cell cytolytic response | ||||
| No major side effects during long-term follow-up | ||||
| Mudrabettu et al[175] | Kidney transplant | NCT02409940 | Autologous BM-MSCs | MSC infusion was safe |
| Increment in proliferation of regulatory T cells | ||||
| Reduction in proliferation of CD4+ T cell | ||||
| Pan et al[183] | Kidney transplant | NA | Autologous BM-MSCs | None of the MSC recipients experienced immediate or long-term toxicity from the treatment |
| Comparable incidence of acute rejection and similar graft function and survival between control and study groups | ||||
| MSCs permitted the use of lower dosages of nephrotoxic calcineurin inhibitors |
The first few trials using MSCs from different tissue sources (bone marrow, adipose, umbilical cord, etc.) either autologously or allogeneically suggested that these cells can be given safely to humans. However, the efficacies of MSCs to treat kidney diseases have mixed results. In one study, patients who had a high risk of post-operative AKI and underwent cardiac surgery, concurrently received allogeneic BM-MSCs. The patients had a shorter hospital stay and did not need readmission. The administered BM-MSCs did not have adverse effects and protected the patients against early and late post-surgery kidney function deterioration[159,160]. However, Swaminathan et al[161] reported a contrasting finding, whereby the administration of allogeneic MSCs did not decrease the time to recovery of kidney function in patients with a developed stage of AKI after cardiac surgery. They also did not detect any significant differences between the group treated with allogeneic MSCs and the group treated with placebo in the 30-day all-cause mortality study. While the rates of adverse events did not differ between groups, the MSC infusion was safe and well-tolerated[161].
More human trials have been conducted using MSCs to treat CKD. A pilot study assessing the safety and clinical feasibility of autologous administration of AD-MSCs for patients with CKD reported that the cells were safe and did not exert any adverse effects[162]. At the same time, improvement in urinary protein excretion was observed[162]. However, Makhlough et al[163,164] reported no significant changes in estimated glomerular filtration rate and SCr during the 18 mo of follow-up after autologous BM-MSCs were transplanted in these CKD patients.
In studies involving the treatment of autoimmune diseases such as systemic lupus erythematosus (SLE) with MSCs, the cells were investigated for their therapeutic benefits. Sun L and team conducted phase I/II clinical trials to examine the effects of allogeneic BM-MSCs and UC-MSCs infusions in patients with primary and refractory SLE[165-167]. They found that the infusion of either allogeneic BM-MSCs or UC-MSCs was safe and well-tolerated. Besides improving the SLE Disease Activity Index and kidney function, the level of proteinuria declined 24 h after the MSCs transplantation[168]. Furthermore, among the SLE patients, allogeneic MSCs transplantation resulted in renal remission for active LN patients within a 12-mo follow-up period[169]. Barbado et al[170] in their pilot study also reported a dramatic improvement to proteinuria level during the first month post-treatment. The ameliorations were sustained throughout the follow-up period of nine months[170]. Conversely, a recent multicentre randomised, double-blind controlled trial showed that UC-MSCs have no apparent additional effects over and above standard immunosuppression therapy[171].
Given the immunomodulatory properties of MSCs, these cells have been transplanted in patients who received kidney transplants to promote immune tolerance to kidney transplantation in the setting of peri-transplant T cell-depleting induction therapy. Reports showed that transplanted MSCs were safe and without major side effects even over a long-term follow-up[172]. Additionally, there was increased proliferation of Treg noted[173,174], increment in regulatory T cell proportion[175], and improved early allograft function[176]. Simultaneously, the transplanted MSCs also controlled memory CD8+ T cells’ functions and reduced donor-specific CD8+ T cell cytolitic response[173]. Furthermore, MSCs infusion showed a lower incidence of acute rejection leading to a decreased risk of opport
A recent phase I trial using autologous CD34+ EPCs in treating CKD at stage III or IV has been reported. Yip HK and team demonstrated that intra-renal arterial transfusion of CD34+ EPCs was safe and well-tolerated[178]. However, when the efficacy in a phase II randomised controlled trial was further investigated, the infused EPCs did not offer additional benefit to patients with CKD up to a follow-up period of 12 months. Despite the less encouraging outcome, it is worth noting that the unfavourable clinical outcomes, such as dialysis or death, were significantly lower in the treatment group than those in the control group[179]. Currently, many clinical trials are still on-going and will provide more insights into and possibly further support these achievements with cell-based therapy for kidney diseases.
Although stem cell therapies in kidney regeneration from in vitro and preclinical studies are promising, and an encouraging safety profile have been demonstrated in early-phased human clinical trials, these cell-based therapies are yet to be translated into more significant proof of clinical efficacy. The side effects of stem cell therapies on kidney diseases still need further investigation, as the preliminary results available still lack long-term follow-up data. Some concerns about the use of live stem cells should be kept in consideration. EVs should also be evaluated as a possible alternative to live stem cells. The use of stem cells-derived EVs that can mimic its parental cells' effects in renoprotection could be pursued. Nevertheless, it appears that stem cell therapy will have a great future in the field of kidney regeneration. Further clarification will be gained on the stem cells' protective mechanisms in the treatment of kidney diseases through further understanding of the mechanisms of stem cells’ actions in vivo. The success of this new cell-based therapy could genuinely change the scope of the future of regenerative medicine.
Manuscript source: Invited manuscript
Specialty type: Cell biology
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME, Saleh F, Wahid M S-Editor: Liu M L-Editor: A P-Editor: Liu JH
| 1. | Carney EF. The impact of chronic kidney disease on global health. Nat Rev Nephrol. 2020;16:251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 146] [Article Influence: 36.5] [Reference Citation Analysis (1)] |
| 2. | GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2617] [Cited by in F6Publishing: 2488] [Article Influence: 622.0] [Reference Citation Analysis (0)] |
| 3. | Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96:414-422D. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 386] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 4. | Luyckx VA, Al-Aly Z, Bello AK, Bellorin-Font E, Carlini RG, Fabian J, Garcia-Garcia G, Iyengar A, Sekkarie M, van Biesen W, Ulasi I, Yeates K, Stanifer J. Sustainable Development Goals relevant to kidney health: an update on progress. Nat Rev Nephrol. 2021;17:15-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 5. | Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975-1982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1175] [Cited by in F6Publishing: 1207] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 6. | Vanholder R, Lameire N, Annemans L, Van Biesen W. Cost of renal replacement: how to help as many as possible while keeping expenses reasonable? Nephrol Dial Transplant. 2016;31:1251-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Saidi RF, Hejazii Kenari SK. Challenges of organ shortage for transplantation: solutions and opportunities. Int J Organ Transplant Med. 2014;5:87-96. [PubMed] [Cited in This Article: ] |
| 8. | Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldaña HA. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch Med Res. 2021;52:93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 9. | Chin SP, Maskon O, Tan CS, Anderson JE, Wong CY, Hassan HHC, Choor CK, Fadilah SAW, Cheong SK. Synergistic effects of intracoronary infusion of autologous bone marrow-derived mesenchymal stem cells and revascularization procedure on improvement of cardiac function in patients with severe ischemic cardiomyopathy. Stem Cell Investig. 2021;8:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, Alturki M, Fallatah M, Almalik A, Alhasan AH, Shah K, Karp JM. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6:eaba6884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 298] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 11. | Steenhard BM, Isom KS, Cazcarro P, Dunmore JH, Godwin AR, St John PL, Abrahamson DR. Integration of embryonic stem cells in metanephric kidney organ culture. J Am Soc Nephrol. 2005;16:1623-1631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Narayanan K, Schumacher KM, Tasnim F, Kandasamy K, Schumacher A, Ni M, Gao S, Gopalan B, Zink D, Ying JY. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int. 2013;83:593-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 14. | Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16:3527-3534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 178] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 15. | Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338-2347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 16. | Vazquez-Zapien GJ, Martinez-Cuazitl A, Rangel-Cova LS, Camacho-Ibarra A, Mata-Miranda MM. Biochemical and histological effects of embryonic stem cells in a mouse model of renal failure. Rom J Morphol Embryol. 2019;60:189-194. [PubMed] [Cited in This Article: ] |
| 17. | Liu D, Cheng F, Pan S, Liu Z. Stem cells: a potential treatment option for kidney diseases. Stem Cell Res Ther. 2020;11:249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Geuens T, van Blitterswijk CA, LaPointe VLS. Overcoming kidney organoid challenges for regenerative medicine. NPJ Regen Med. 2020;5:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Tan Z, Shan J, Rak-Raszewska A, Vainio SJ. Embryonic Stem Cells Derived Kidney Organoids as Faithful Models to Target Programmed Nephrogenesis. Sci Rep. 2018;8:16618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Li SC, Zhong JF. Twisting immune responses for allogeneic stem cell therapy. World J Stem Cells. 2009;1:30-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Chhabra P, Brayman KL. The use of stem cells in kidney disease. Curr Opin Organ Transplant. 2009;14:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17989] [Cited by in F6Publishing: 17040] [Article Influence: 946.7] [Reference Citation Analysis (0)] |
| 23. | Erdlenbruch F, Rohwedel J, Ralfs P, Thomitzek A, Kramer J, Cakiroglu F. Generation of induced pluripotent stem cells (iPSCs) from human foreskin fibroblasts. Stem Cell Res. 2018;33:79-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Fernandes S, Shinde P, Khan N, Singh S, Vardhan S, Nair V, Kale V, Limaye L. Derivation of human iPSC line NCCSi002-A from umbilical cord blood (UCB) CD34+cells of donor from Indian ethnicity. Stem Cell Res. 2018;26:80-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Kim Y, Rim YA, Yi H, Park N, Park SH, Ju JH. The Generation of Human Induced Pluripotent Stem Cells from Blood Cells: An Efficient Protocol Using Serial Plating of Reprogrammed Cells by Centrifugation. Stem Cells Int. 2016;2016:1329459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Vlahos K, Sourris K, Mayberry R, McDonald P, Bruveris FF, Schiesser JV, Bozaoglu K, Lockhart PJ, Stanley EG, Elefanty AG. Generation of iPSC lines from peripheral blood mononuclear cells from 5 healthy adults. Stem Cell Res. 2019;34:101380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Re S, Dogan AA, Ben-Shachar D, Berger G, Werling AM, Walitza S, Grünblatt E. Improved Generation of Induced Pluripotent Stem Cells From Hair Derived Keratinocytes - A Tool to Study Neurodevelopmental Disorders as ADHD. Front Cell Neurosci. 2018;12:321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Song B, Niclis JC, Alikhan MA, Sakkal S, Sylvain A, Kerr PG, Laslett AL, Bernard CA, Ricardo SD. Generation of induced pluripotent stem cells from human kidney mesangial cells. J Am Soc Nephrol. 2011;22:1213-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Zhou T, Benda C, Dunzinger S, Huang Y, Ho JC, Yang J, Wang Y, Zhang Y, Zhuang Q, Li Y, Bao X, Tse HF, Grillari J, Grillari-Voglauer R, Pei D, Esteban MA. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7:2080-2089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 30. | Steinle H, Weber M, Behring A, Mau-Holzmann U, von Ohle C, Popov AF, Schlensak C, Wendel HP, Avci-Adali M. Reprogramming of Urine-Derived Renal Epithelial Cells into iPSCs Using srRNA and Consecutive Differentiation into Beating Cardiomyocytes. Mol Ther Nucleic Acids. 2019;17:907-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Su J, Wang J, Wang L, Li T, Wang H, Shen J, Zhang J, Lin W, Huang J, Liang P. Generation of five induced pluripotent stem cell lines with DMD/c.497G > T mutation from renal epithelial cells of a Duchenne muscular dystrophy patient and a recessive carrier parent. Stem Cell Res. 2020;49:102021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Mae SI, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, Arai S, Sato-Otubo A, Toyoda T, Takahashi K, Nakayama N, Cowan CA, Aoi T, Ogawa S, McMahon AP, Yamanaka S, Osafune K. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun. 2013;4:1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 33. | Araoka T, Mae S, Kurose Y, Uesugi M, Ohta A, Yamanaka S, Osafune K. Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PLoS One. 2014;9:e84881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, Bonventre JV. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. 2014;25:1211-1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 209] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 35. | Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 558] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 36. | Toyohara T, Mae S, Sueta S, Inoue T, Yamagishi Y, Kawamoto T, Kasahara T, Hoshina A, Toyoda T, Tanaka H, Araoka T, Sato-Otsubo A, Takahashi K, Sato Y, Yamaji N, Ogawa S, Yamanaka S, Osafune K. Cell Therapy Using Human Induced Pluripotent Stem Cell-Derived Renal Progenitors Ameliorates Acute Kidney Injury in Mice. Stem Cells Transl Med. 2015;4:980-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | Lee PY, Chien Y, Chiou GY, Lin CH, Chiou CH, Tarng DC. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplant. 2012;21:2569-2585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Tarng DC, Tseng WC, Lee PY, Chiou SH, Hsieh SL. Induced Pluripotent Stem Cell-Derived Conditioned Medium Attenuates Acute Kidney Injury by Downregulating the Oxidative Stress-Related Pathway in Ischemia-Reperfusion Rats. Cell Transplant. 2016;25:517-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Gupta N, Dilmen E, Morizane R. 3D kidney organoids for bench-to-bedside translation. J Mol Med (Berl). 2021;99:477-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Grassi L, Alfonsi R, Francescangeli F, Signore M, De Angelis ML, Addario A, Costantini M, Flex E, Ciolfi A, Pizzi S, Bruselles A, Pallocca M, Simone G, Haoui M, Falchi M, Milella M, Sentinelli S, Di Matteo P, Stellacci E, Gallucci M, Muto G, Tartaglia M, De Maria R, Bonci D. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 2019;10:201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 41. | Romero-Guevara R, Ioannides A, Xinaris C. Kidney Organoids as Disease Models: Strengths, Weaknesses and Perspectives. Front Physiol. 2020;11:563981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, Yong KSM, Chen Q, Campistol JM, Fang M, Khor CC, Foo JN, Izpisua Belmonte JC, Xia Y. Generation of Human PSC-Derived Kidney Organoids with Patterned Nephron Segments and a De Novo Vascular Network. Cell Stem Cell. 2019;25:373-387.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 43. | Benedetti V, Brizi V, Guida P, Tomasoni S, Ciampi O, Angeli E, Valbusa U, Benigni A, Remuzzi G, Xinaris C. Engineered Kidney Tubules for Modeling Patient-Specific Diseases and Drug Discovery. EBioMedicine. 2018;33:253-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Takasato M, Little MH. Making a Kidney Organoid Using the Directed Differentiation of Human Pluripotent Stem Cells. Methods Mol Biol. 2017;1597:195-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, Wilson S, Quinlan C, Ho G, Holman K, Bennetts B, Crawford J, Trnka P, Oshlack A, Patel C, Mallett A, Simons C, Little MH. Patient-iPSC-Derived Kidney Organoids Show Functional Validation of a Ciliopathic Renal Phenotype and Reveal Underlying Pathogenetic Mechanisms. Am J Hum Genet. 2018;102:816-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 46. | Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 904] [Cited by in F6Publishing: 928] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 47. | Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33:1193-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 531] [Cited by in F6Publishing: 566] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 48. | Hu Q, Friedrich AM, Johnson LV, Clegg DO. Memory in induced pluripotent stem cells: reprogrammed human retinal-pigmented epithelial cells show tendency for spontaneous redifferentiation. Stem Cells. 2010;28:1981-1991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 49. | Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 919] [Cited by in F6Publishing: 883] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 50. | Pfaff N, Lachmann N, Kohlscheen S, Sgodda M, Araúzo-Bravo MJ, Greber B, Kues W, Glage S, Baum C, Niemann H, Schambach A, Cantz T, Moritz T. Efficient hematopoietic redifferentiation of induced pluripotent stem cells derived from primitive murine bone marrow cells. Stem Cells Dev. 2012;21:689-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Ozkok A, Yildiz A. Endothelial Progenitor Cells and Kidney Diseases. Kidney Blood Press Res. 2018;43:701-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Chopra H, Hung MK, Kwong DL, Zhang CF, Pow EHN. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018;2018:9847015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 53. | Pang P, Abbott M, Chang SL, Abdi M, Chauhan N, Mistri M, Ghofrani J, Fucci QA, Walker C, Leonardi C, Grady S, Halim A, Hoffman R, Lu T, Cao H, Tullius SG, Malek S, Kumar S, Steele G, Kibel A, Freedman BS, Waikar SS, Siedlecki AM. Human vascular progenitor cells derived from renal arteries are endothelial-like and assist in the repair of injured renal capillary networks. Kidney Int. 2017;91:129-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Patschan D, Schwarze K, Henze E, Patschan S, Müller GA. The endothelial-to-mesenchymal transition and endothelial cilia in EPC-mediated postischemic kidney protection. Am J Physiol Renal Physiol. 2016;310:F679-F687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Sangidorj O, Yang SH, Jang HR, Lee JP, Cha RH, Kim SM, Lim CS, Kim YS. Bone marrow-derived endothelial progenitor cells confer renal protection in a murine chronic renal failure model. Am J Physiol Renal Physiol. 2010;299:F325-F335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Huang TH, Chen YT, Sung PH, Chiang HJ, Chen YL, Chai HT, Chung SY, Tsai TH, Yang CC, Chen CH, Chang HW, Sun CK, Yip HK. Peripheral blood-derived endothelial progenitor cell therapy prevented deterioration of chronic kidney disease in rats. Am J Transl Res. 2015;7:804-824. [PubMed] [Cited in This Article: ] |
| 57. | Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 58. | Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells. 2010;28:1039-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Ebrahimi B, Li Z, Eirin A, Zhu XY, Textor SC, Lerman LO. Addition of endothelial progenitor cells to renal revascularization restores medullary tubular oxygen consumption in swine renal artery stenosis. Am J Physiol Renal Physiol. 2012;302:F1478-F1485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83-92. [PubMed] [Cited in This Article: ] |
| 61. | Mahmoudifar N, Doran PM. Mesenchymal Stem Cells Derived from Human Adipose Tissue. Methods Mol Biol. 2015;1340:53-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ, Han ZC. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017-1026. [PubMed] [Cited in This Article: ] |
| 63. | Choo KB, Tai L, Hymavathee KS, Wong CY, Nguyen PN, Huang CJ, Cheong SK, Kamarul T. Oxidative stress-induced premature senescence in Wharton's jelly-derived mesenchymal stem cells. Int J Med Sci. 2014;11:1201-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 64. | Talwadekar MD, Kale VP, Limaye LS. Placenta-derived mesenchymal stem cells possess better immunoregulatory properties compared to their cord-derived counterparts-a paired sample study. Sci Rep. 2015;5:15784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 65. | Longhini ALF, Salazar TE, Vieira C, Trinh T, Duan Y, Pay LM, Li Calzi S, Losh M, Johnston NA, Xie H, Kim M, Hunt RJ, Yoder MC, Santoro D, McCarrel TM, Grant MB. Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses. PLoS One. 2019;14:e0212642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Spitzhorn LS, Rahman MS, Schwindt L, Ho HT, Wruck W, Bohndorf M, Wehrmeyer S, Ncube A, Beyer I, Hagenbeck C, Balan P, Fehm T, Adjaye J. Isolation and Molecular Characterization of Amniotic Fluid-Derived Mesenchymal Stem Cells Obtained from Caesarean Sections. Stem Cells Int. 2017;2017:5932706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 67. | Čamernik K, Mihelič A, Mihalič R, Marolt Presen D, Janež A, Trebše R, Marc J, Zupan J. Skeletal-muscle-derived mesenchymal stem/stromal cells from patients with osteoarthritis show superior biological properties compared to bone-derived cells. Stem Cell Res. 2019;38:101465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34:695-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 69. | Mastrolia I, Foppiani EM, Murgia A, Candini O, Samarelli AV, Grisendi G, Veronesi E, Horwitz EM, Dominici M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl Med. 2019;8:1135-1148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 70. | Rota C, Morigi M, Imberti B. Stem Cell Therapies in Kidney Diseases: Progress and Challenges. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 71. | Wang Y, He J, Pei X, Zhao W. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology (Carlton). 2013;18:201-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 72. | Sávio-Silva C, Soinski-Sousa PE, Balby-Rocha MTA, Lira ÁO, Rangel ÉB. Mesenchymal stem cell therapy in acute kidney injury (AKI): review and perspectives. Rev Assoc Med Bras (1992). 2020;66 Suppl 1:s45-s54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Papazova DA, Oosterhuis NR, Gremmels H, van Koppen A, Joles JA, Verhaar MC. Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech. 2015;8:281-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 74. | Zhao L, Han F, Wang J, Chen J. Current understanding of the administration of mesenchymal stem cells in acute kidney injury to chronic kidney disease transition: a review with a focus on preclinical models. Stem Cell Res Ther. 2019;10:385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Brasile L, Henry N, Orlando G, Stubenitsky B. Potentiating Renal Regeneration Using Mesenchymal Stem Cells. Transplantation. 2019;103:307-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Ardeshirylajimi A, Vakilian S, Salehi M, Mossahebi-Mohammadi M. Renal Differentiation of Mesenchymal Stem Cells Seeded on Nanofibrous Scaffolds Improved by Human Renal Tubular Cell Lines-Conditioned Medium. ASAIO J. 2017;63:356-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035-1041. [PubMed] [Cited in This Article: ] |
| 78. | Bussolati B, Hauser PV, Carvalhosa R, Camussi G. Contribution of stem cells to kidney repair. Curr Stem Cell Res Ther. 2009;4:2-8. [PubMed] [Cited in This Article: ] |
| 79. | Yuan L, Liu HQ, Wu MJ. Human embryonic mesenchymal stem cells participate in differentiation of renal tubular cells in newborn mice. Exp Ther Med. 2016;12:641-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Baer PC, Bereiter-Hahn J, Missler C, Brzoska M, Schubert R, Gauer S, Geiger H. Conditioned medium from renal tubular epithelial cells initiates differentiation of human mesenchymal stem cells. Cell Prolif. 2009;42:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Wong CY, Chang YM, Tsai YS, Ng WV, Cheong SK, Chang TY, Chung IF, Lim YM. Decoding the differentiation of mesenchymal stem cells into mesangial cells at the transcriptomic level. BMC Genomics. 2020;21:467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Wong CY, Tan EL, Cheong SK. In vitro differentiation of mesenchymal stem cells into mesangial cells when co-cultured with injured mesangial cells. Cell Biol Int. 2014;38:497-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 83. | Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G, Zhu Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res. 2016;8:4289-4299. [PubMed] [Cited in This Article: ] |
| 84. | Wang C, Li Y, Yang M, Zou Y, Liu H, Liang Z, Yin Y, Niu G, Yan Z, Zhang B. Efficient Differentiation of Bone Marrow Mesenchymal Stem Cells into Endothelial Cells in Vitro. Eur J Vasc Endovasc Surg. 2018;55:257-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 85. | Zhou S, Qiao YM, Liu YG, Liu D, Hu JM, Liao J, Li M, Guo Y, Fan LP, Li LY, Zhao M. Bone marrow derived mesenchymal stem cells pretreated with erythropoietin accelerate the repair of acute kidney injury. Cell Biosci. 2020;10:130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Geng Y, Zhang L, Fu B, Zhang J, Hong Q, Hu J, Li D, Luo C, Cui S, Zhu F, Chen X. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res Ther. 2014;5:80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 87. | Zoja C, Garcia PB, Rota C, Conti S, Gagliardini E, Corna D, Zanchi C, Bigini P, Benigni A, Remuzzi G, Morigi M. Mesenchymal stem cell therapy promotes renal repair by limiting glomerular podocyte and progenitor cell dysfunction in adriamycin-induced nephropathy. Am J Physiol Renal Physiol. 2012;303:F1370-F1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 88. | Wong CY, Cheong SK, Mok PL, Leong CF. Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model. Pathology. 2008;40:52-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Kunter U, Rong S, Djuric Z, Boor P, Müller-Newen G, Yu D, Floege J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17:2202-2212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 90. | Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794-1804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 519] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 91. | Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, Rambaldi A, Remuzzi A, Remuzzi G. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075-2082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 309] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 92. | Qian H, Yang H, Xu W, Yan Y, Chen Q, Zhu W, Cao H, Yin Q, Zhou H, Mao F, Chen Y. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22:325-332. [PubMed] [Cited in This Article: ] |
| 93. | Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 94. | Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31-F42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 894] [Cited by in F6Publishing: 858] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 95. | Sherif IO, Al-Mutabagani LA, Alnakhli AM, Sobh MA, Mohammed HE. Renoprotective effects of angiotensin receptor blocker and stem cells in acute kidney injury: Involvement of inflammatory and apoptotic markers. Exp Biol Med (Maywood). 2015;240:1572-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Eirin A, Lerman LO. Mesenchymal stem cell treatment for chronic renal failure. Stem Cell Res Ther. 2014;5:83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 97. | Zhuang Q, Ma R, Yin Y, Lan T, Yu M, Ming Y. Mesenchymal Stem Cells in Renal Fibrosis: The Flame of Cytotherapy. Stem Cells Int. 2019;2019:8387350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 98. | Choi S, Park M, Kim J, Hwang S, Park S, Lee Y. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Choi SJ, Kim JK, Hwang SD. Mesenchymal stem cell therapy for chronic renal failure. Expert Opin Biol Ther. 2010;10:1217-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 100. | Missoum A. Recent Updates on Mesenchymal Stem Cell Based Therapy for Acute Renal Failure. Curr Urol. 2020;13:189-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 101. | Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 257] [Article Influence: 21.4] [Reference Citation Analysis (1)] |
| 102. | Nargesi AA, Lerman LO, Eirin A. Mesenchymal Stem Cell-derived Extracellular Vesicles for Renal Repair. Curr Gene Ther. 2017;17:29-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 103. | Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 931] [Cited by in F6Publishing: 966] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 104. | Bank JR, Rabelink TJ, de Fijter JW, Reinders ME. Safety and Efficacy Endpoints for Mesenchymal Stromal Cell Therapy in Renal Transplant Recipients. J Immunol Res. 2015;2015:391797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 105. | Pool M, Leuvenink H, Moers C. Reparative and Regenerative Effects of Mesenchymal Stromal Cells-Promising Potential for Kidney Transplantation? Int J Mol Sci. 2019;20:4614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | De Martino M, Zonta S, Rampino T, Gregorini M, Frassoni F, Piotti G, Bedino G, Cobianchi L, Dal Canton A, Dionigi P, Alessiani M. Mesenchymal stem cells infusion prevents acute cellular rejection in rat kidney transplantation. Transplant Proc. 2010;42:1331-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 107. | Yu P, Wang Z, Liu Y, Xiao Z, Guo Y, Li M, Zhao M. Marrow Mesenchymal Stem Cells Effectively Reduce Histologic Changes in a Rat Model of Chronic Renal Allograft Rejection. Transplant Proc. 2017;49:2194-2203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 108. | Franquesa M, Herrero E, Torras J, Ripoll E, Flaquer M, Gomà M, Lloberas N, Anegon I, Cruzado JM, Grinyó JM, Herrero-Fresneda I. Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev. 2012;21:3125-3135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 109. | Peired AJ, Sisti A, Romagnani P. Mesenchymal Stem Cell-Based Therapy for Kidney Disease: A Review of Clinical Evidence. Stem Cells Int. 2016;2016:4798639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 110. | Perico N, Casiraghi F, Remuzzi G. Clinical Translation of Mesenchymal Stromal Cell Therapies in Nephrology. J Am Soc Nephrol. 2018;29:362-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 111. | Bochon B, Kozubska M, Surygała G, Witkowska A, Kuźniewicz R, Grzeszczak W, Wystrychowski G. Mesenchymal Stem Cells-Potential Applications in Kidney Diseases. Int J Mol Sci. 2019;20:2462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 112. | Ashour RH, Saad MA, Sobh MA, Al-Husseiny F, Abouelkheir M, Awad A, Elghannam D, Abdel-Ghaffar H, Sobh M. Comparative study of allogenic and xenogeneic mesenchymal stem cells on cisplatin-induced acute kidney injury in Sprague-Dawley rats. Stem Cell Res Ther. 2016;7:126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 113. | Kim JH, Park DJ, Yun JC, Jung MH, Yeo HD, Kim HJ, Kim DW, Yang JI, Lee GW, Jeong SH, Roh GS, Chang SH. Human adipose tissue-derived mesenchymal stem cells protect kidneys from cisplatin nephrotoxicity in rats. Am J Physiol Renal Physiol. 2012;302:F1141-F1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 114. | Katsuno T, Ozaki T, Saka Y, Furuhashi K, Kim H, Yasuda K, Yamamoto T, Sato W, Tsuboi N, Mizuno M, Ito Y, Imai E, Matsuo S, Maruyama S. Low serum cultured adipose tissue-derived stromal cells ameliorate acute kidney injury in rats. Cell Transplant. 2013;22:287-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 115. | Musiał-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 231] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 116. | Bárcia RN, Santos JM, Filipe M, Teixeira M, Martins JP, Almeida J, Água-Doce A, Almeida SC, Varela A, Pohl S, Dittmar KE, Calado S, Simões SI, Gaspar MM, Cruz ME, Lindenmaier W, Graça L, Cruz H, Cruz PE. What Makes Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells Superior Immunomodulators When Compared to Bone Marrow Derived Mesenchymal Stromal Cells? Stem Cells Int. 2015;2015:583984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 117. | Fazzina R, Iudicone P, Fioravanti D, Bonanno G, Totta P, Zizzari IG, Pierelli L. Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues. Stem Cell Res Ther. 2016;7:122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 118. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1018] [Cited by in F6Publishing: 1115] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 119. | La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasà L, Cappello F, Zummo G, Farina F. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009;131:267-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 120. | Jang HR, Park JH, Kwon GY, Lee JE, Huh W, Jin HJ, Choi SJ, Oh W, Oh HY, Kim YG. Effect of preemptive treatment with human umbilical cord blood-derived mesenchymal stem cells on the development of renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2014;307:F1149-F1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Rodrigues CE, Capcha JM, de Bragança AC, Sanches TR, Gouveia PQ, de Oliveira PA, Malheiros DM, Volpini RA, Santinho MA, Santana BA, Calado RD, Noronha IL, Andrade L. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res Ther. 2017;8:19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 122. | Li W, Wang L, Chu X, Cui H, Bian Y. Icariin combined with human umbilical cord mesenchymal stem cells significantly improve the impaired kidney function in chronic renal failure. Mol Cell Biochem. 2017;428:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 123. | Xiang E, Han B, Zhang Q, Rao W, Wang Z, Chang C, Zhang Y, Tu C, Li C, Wu D. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res Ther. 2020;11:336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 124. | Morigi M, Rota C, Montemurro T, Montelatici E, Lo Cicero V, Imberti B, Abbate M, Zoja C, Cassis P, Longaretti L, Rebulla P, Introna M, Capelli C, Benigni A, Remuzzi G, Lazzari L. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28:513-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 125. | Hauser PV, De Fazio R, Bruno S, Sdei S, Grange C, Bussolati B, Benedetto C, Camussi G. Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am J Pathol. 2010;177:2011-2021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 126. | George SK, Abolbashari M, Kim TH, Zhang C, Allickson J, Jackson JD, Lee SJ, Ko IK, Atala A, Yoo JJ. Effect of Human Amniotic Fluid Stem Cells on Kidney Function in a Model of Chronic Kidney Disease. Tissue Eng Part A. 2019;25:1493-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 127. | Sedrakyan S, Da Sacco S, Milanesi A, Shiri L, Petrosyan A, Varimezova R, Warburton D, Lemley KV, De Filippo RE, Perin L. Injection of amniotic fluid stem cells delays progression of renal fibrosis. J Am Soc Nephrol. 2012;23:661-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 128. | Cetinkaya B, Unek G, Kipmen-Korgun D, Koksoy S, Korgun ET. Effects of Human Placental Amnion Derived Mesenchymal Stem Cells on Proliferation and Apoptosis Mechanisms in Chronic Kidney Disease in the Rat. Int J Stem Cells. 2019;12:151-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 129. | Liu J, Lu X, Lou Y, Cai Y, Cui W, Wang J, Nie P, Chen L, Li B, Luo P. Xenogeneic Transplantation of Human Placenta-Derived Mesenchymal Stem Cells Alleviates Renal Injury and Reduces Inflammation in a Mouse Model of Lupus Nephritis. Biomed Res Int. 2019;2019:9370919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 130. | Tang TT, Lv LL, Lan HY, Liu BC. Extracellular Vesicles: Opportunities and Challenges for the Treatment of Renal Diseases. Front Physiol. 2019;10:226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 131. | Kwon SH. Extracellular vesicles in renal physiology and clinical applications for renal disease. Korean J Intern Med. 2019;34:470-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27:3037-3042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 319] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 133. | Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 548] [Cited by in F6Publishing: 713] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 134. | Park KS, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 135. | Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23:812-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 625] [Cited by in F6Publishing: 751] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 136. | Grange C, Skovronova R, Marabese F, Bussolati B. Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 137. | Karpman D, Ståhl AL, Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol. 2017;13:545-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 138. | Collino F, Bruno S, Incarnato D, Dettori D, Neri F, Provero P, Pomatto M, Oliviero S, Tetta C, Quesenberry PJ, Camussi G. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J Am Soc Nephrol. 2015;26:2349-2360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 139. | Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474-1483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 589] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 140. | Zhang G, Zou X, Miao S, Chen J, Du T, Zhong L, Ju G, Liu G, Zhu Y. The anti-oxidative role of micro-vesicles derived from human Wharton-Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS One. 2014;9:e92129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 141. | Kholia S, Herrera Sanchez MB, Cedrino M, Papadimitriou E, Tapparo M, Deregibus MC, Bruno S, Antico F, Brizzi MF, Quesenberry PJ, Camussi G. Mesenchymal Stem Cell Derived Extracellular Vesicles Ameliorate Kidney Injury in Aristolochic Acid Nephropathy. Front Cell Dev Biol. 2020;8:188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 142. | Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 225] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 143. | Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 400] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 144. | Cantaluppi V, Medica D, Mannari C, Stiaccini G, Figliolini F, Dellepiane S, Quercia AD, Migliori M, Panichi V, Giovannini L, Bruno S, Tetta C, Biancone L, Camussi G. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol Dial Transplant. 2015;30:410-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 145. | Collino F, Lopes JA, Tapparo M, Tortelote GG, Kasai-Brunswick TH, Lopes GMC, Almeida DB, Skovronova R, Wendt CHC, Miranda KR, Bussolati B, Vieyra A, Lindoso RS. Extracellular Vesicles Derived from Induced Pluripotent Stem Cells Promote Renoprotection in Acute Kidney Injury Model. Cells. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 146. | Yuan X, Li D, Chen X, Han C, Xu L, Huang T, Dong Z, Zhang M. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 2017;8:3200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 147. | Grange C, Bellucci L, Bussolati B, Ranghino A. Potential Applications of Extracellular Vesicles in Solid Organ Transplantation. Cells. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 148. | Wu X, Yan T, Wang Z, Wu X, Cao G, Zhang C, Tian X, Wang J. Micro-vesicles derived from human Wharton's Jelly mesenchymal stromal cells mitigate renal ischemia-reperfusion injury in rats after cardiac death renal transplantation. J Cell Biochem. 2018;119:1879-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 149. | Gregorini M, Corradetti V, Pattonieri EF, Rocca C, Milanesi S, Peloso A, Canevari S, De Cecco L, Dugo M, Avanzini MA, Mantelli M, Maestri M, Esposito P, Bruno S, Libetta C, Dal Canton A, Rampino T. Perfusion of isolated rat kidney with Mesenchymal Stromal Cells/Extracellular Vesicles prevents ischaemic injury. J Cell Mol Med. 2017;21:3381-3393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 150. | Bao YW, Yuan Y, Chen JH, Lin WQ. Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res. 2018;39:72-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 151. | van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 861] [Cited by in F6Publishing: 824] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 152. | Akhtar A. The flaws and human harms of animal experimentation. Camb Q Healthc Ethics. 2015;24:407-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 205] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 153. | Hewitson TD, Holt SG, Smith ER. Animal Models to Study Links between Cardiovascular Disease and Renal Failure and Their Relevance to Human Pathology. Front Immunol. 2015;6:465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 154. | Amarasiri SS, Attanayake AP, Jayatilaka KAPW, Mudduwa LKB. Animal models of chronic kidney disease: Screening tool to investigate nephroprotective effects of natural products. Int J Pharm Chem Anal. 2018;5:52-58. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 155. | Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philos Ethics Humanit Med. 2009;4:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 454] [Cited by in F6Publishing: 412] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 156. | Togoe EB, Silva IS, Cury JL, Souza AS, Borges JH, Saturnino KC. Animal model of chronic kidney disease using a unilateral technique of renal ischemia and reperfusion in White New Zealand rabbits. Acta Cir Bras. 2014;29:651-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 157. | Becker GJ, Hewitson TD. Animal models of chronic kidney disease: useful but not perfect. Nephrol Dial Transplant. 2013;28:2432-2438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 158. | Packialakshmi B, Stewart IJ, Burmeister DM, Chung KK, Zhou X. Large animal models for translational research in acute kidney injury. Ren Fail. 2020;42:1042-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 159. | Tögel FE, Westenfelder C. Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis. 2012;60:1012-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 160. | Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl (2011). 2011;1:103-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 161. | Swaminathan M, Stafford-Smith M, Chertow GM, Warnock DG, Paragamian V, Brenner RM, Lellouche F, Fox-Robichaud A, Atta MG, Melby S, Mehta RL, Wald R, Verma S, Mazer CD; ACT-AKI investigators. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J Am Soc Nephrol. 2018;29:260-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 162. | Villanueva S, González F, Lorca E, Tapia A, López VG, Strodthoff R, Fajre F, Carreño JE, Valjalo R, Vergara C, Lecanda M, Bartolucci J, Figueroa FE, Khoury M. Adipose tissue-derived mesenchymal stromal cells for treating chronic kidney disease: A pilot study assessing safety and clinical feasibility. Kidney Res Clin Pract. 2019;38:176-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 163. | Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Hosseini SE, Jaroughi N, Bolurieh T, Baharvand H, Aghdami N. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8:116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 164. | Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Dastgheib M, Janbabaee G, Hosseini SE, Falah N, Abbasi F, Baharvand H, Aghdami N. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: A safety study with 18 months of follow-up. Cytotherapy. 2018;20:660-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 165. | Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 433] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 166. | Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, Hou Y, Zeng X, Gilkeson GS, Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 167. | Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467-2475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 318] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 168. | Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S, Sun L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16:R79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 169. | Gu F, Wang D, Zhang H, Feng X, Gilkeson GS, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin Rheumatol. 2014;33:1611-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 170. | Barbado J, Tabera S, Sánchez A, García-Sancho J. Therapeutic potential of allogeneic mesenchymal stromal cells transplantation for lupus nephritis. Lupus. 2018;27:2161-2165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 171. | Deng D, Zhang P, Guo Y, Lim TO. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76:1436-1439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 172. | Perico N, Casiraghi F, Todeschini M, Cortinovis M, Gotti E, Portalupi V, Mister M, Gaspari F, Villa A, Fiori S, Introna M, Longhi E, Remuzzi G. Long-Term Clinical and Immunological Profile of Kidney Transplant Patients Given Mesenchymal Stromal Cell Immunotherapy. Front Immunol. 2018;9:1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 173. | Perico N, Casiraghi F, Gotti E, Introna M, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, Noris M, Remuzzi G. Mesenchymal stromal cells and kidney transplantation: pretransplant infusion protects from graft dysfunction while fostering immunoregulation. Transpl Int. 2013;26:867-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 174. | Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, Marasà M, Golay J, Noris M, Remuzzi G. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6:412-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 175. | Mudrabettu C, Kumar V, Rakha A, Yadav AK, Ramachandran R, Kanwar DB, Nada R, Minz M, Sakhuja V, Marwaha N, Jha V. Safety and efficacy of autologous mesenchymal stromal cells transplantation in patients undergoing living donor kidney transplantation: a pilot study. Nephrology (Carlton). 2015;20:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 176. | Erpicum P, Weekers L, Detry O, Bonvoisin C, Delbouille MH, Grégoire C, Baudoux E, Briquet A, Lechanteur C, Maggipinto G, Somja J, Pottel H, Baron F, Jouret F, Beguin Y. Infusion of third-party mesenchymal stromal cells after kidney transplantation: a phase I-II, open-label, clinical study. Kidney Int. 2019;95:693-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 177. | Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 178. | Lee MS, Lee FY, Chen YL, Sung PH, Chiang HJ, Chen KH, Huang TH, Chiang JY, Yin TC, Chang HW, Yip HK. Investigated the safety of intra-renal arterial transfusion of autologous CD34+ cells and time courses of creatinine levels, endothelial dysfunction biomarkers and micro-RNAs in chronic kidney disease patients-phase I clinical trial. Oncotarget. 2017;8:17750-17762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 179. | Yang CC, Sung PH, Cheng BC, Li YC, Chen YL, Lee MS, Yip HK. Safety and efficacy of intrarenal arterial autologous CD34+ cell transfusion in patients with chronic kidney disease: A randomized, open-label, controlled phase II clinical trial. Stem Cells Transl Med. 2020;9:827-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 180. | Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine. 2016;12:263-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 181. | Saad A, Dietz AB, Herrmann SMS, Hickson LJ, Glockner JF, McKusick MA, Misra S, Bjarnason H, Armstrong AS, Gastineau DA, Lerman LO, Textor SC. Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease. J Am Soc Nephrol. 2017;28:2777-2785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 182. | Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, van Kooten C, Fibbe WE, Rabelink TJ. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. 2013;2:107-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 183. | Pan GH, Chen Z, Xu L, Zhu JH, Xiang P, Ma JJ, Peng YW, Li GH, Chen XY, Fang JL, Guo YH, Zhang L, Liu LS. Low-dose tacrolimus combined with donor-derived mesenchymal stem cells after renal transplantation: a prospective, non-randomized study. Oncotarget. 2016;7:12089-12101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |