Abstract
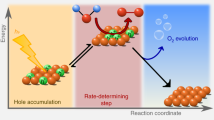
Haematite (α-Fe2O3) has been extensively investigated as a photoanode in photoelectrochemical water oxidation, but the product O2 has a low economic value. Here we expand its applications to the production of value-added chemicals and report its ability to act as a versatile and efficient oxygen atom transfer catalyst under visible-light irradiation. A variety of organic compounds and inorganic anions were successfully oxidized to the corresponding monooxygenation products with high selectivity and Faradaic efficiency by using water as the sole oxygen source. Photoexcited holes generate iron–oxo species (FeIV=O) on α-Fe2O3 surfaces and the process of oxygen-atom transfer is proposed to proceed via a concerted two-hole transfer pathway that involves the transfer of oxygen atoms from the surface FeIV=O to the substrates. The present study proves α-Fe2O3 is an excellent all-inorganic heterogeneous catalyst to drive oxygen atom transfer reactions, and this strategy has significant potential for the synthesis of fine and high-value-added chemicals.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study, which include photoanode preparation, experimental procedures, material characterization, product analysis and computational details are available in the accompanying Supplementary Information or from the authors upon reasonable request. Source data are provided with this paper.
References
Grätzel, M. Photoelectrochemical cells. Nature 414, 338–344 (2001).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Wang, S., Liu, G. & Wang, L. Crystal facet engineering of photoelectrodes for photoelectrochemical water splitting. Chem. Rev. 119, 5192–5247 (2019).
Zeng, Q. et al. Synthesis of WO3/BiVO4 photoanode using a reaction of bismuth nitrate with peroxovanadate on WO3 film for efficient photoelectrocatalytic water splitting and organic pollutant degradation. Appl. Catal. B 217, 21–29 (2017).
Li, T. et al. Photoelectrochemical oxidation of organic substrates in organic media. Nat. Commun. 8, 390 (2017).
Tateno, H., Iguchi, S., Miseki, Y. & Sayama, K. Photo-electrochemical C–H bond activation of cyclohexane using a WO3 photoanode and visible light. Angew. Chem. Int. Ed. 57, 11238–11241 (2018).
Xiong, P. & Xu, H.-C. Chemistry with electrochemically generated N-centered radicals. Acc. Chem. Res. 52, 3339–3350 (2019).
Yuan, Y. & Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution reactions. Acc. Chem. Res. 52, 3309–3324 (2019).
Lum, Y. et al. Tuning OH binding energy enables selective electrochemical oxidation of ethylene to ethylene glycol. Nat. Catal. 3, 14–22 (2020).
Zhang, P., Wang, Y., Li, H. & Antonietti, M. Metal-free oxidation of sulfides by carbon nitride with visible light illumination at room temperature. Green Chem. 14, 1904–1908 (2012).
Zhang, B. et al. Selective oxidation of sulfides on Pt/BiVO4 photocatalyst under visible light irradiation using water as the oxygen source and dioxygen as the electron acceptor. J. Catal. 332, 95–100 (2015).
Zandi, O. & Hamann, T. W. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nat. Chem. 8, 778–783 (2016).
Zhang, Y. et al. Rate-limiting O–O bond formation pathways for water oxidation on hematite photoanode. J. Am. Chem. Soc. 140, 3264–3269 (2018).
Cha, H. G. & Choi, K. S. Combined biomass valorization and hydrogen production in a photoelectrochemical cell. Nat. Chem. 7, 328–333 (2015).
Lhermitte, C. R. & Sivula, K. Alternative oxidation reactions for solar-driven fuel production. ACS Catal. 9, 2007–2017 (2019).
Zhang, L. et al. Photoelectrocatalytic arene C–H amination. Nat. Catal. 2, 266–373 (2019).
Cummings, C. Y., Marken, F., Peter, L. M., Wijayantha, K. G. & Tahir, A. A. New insights into water splitting at mesoporous α-Fe2O3 films: a study by modulated transmittance and impedance spectroscopies. J. Am. Chem. Soc. 134, 1228–1234 (2012).
Chen, L. X., Liu, T., Thurnauer, M. C., Csencsits, R. & Rajh, T. Fe2O3 nanoparticle structures investigated by X-ray absorption near-edge structure, surface modifications, and model calculations. J. Phys. Chem. B 106, 8539–8546 (2002).
Kronawitter, C. X. et al. Electron enrichment in 3d transition metal oxide hetero-nanostructures. Nano Lett. 11, 3855–3861 (2011).
Braun, A. et al. Direct observation of two electron holes in a hematite photoanode during photoelectrochemical water splitting. J. Phys. Chem. C 116, 16870–16875 (2012).
Kumar, A., Goldberg, I., Botoshansky, M., Buchman, Y. & Gross, Z. Oxygen atom transfer reactions from isolated (oxo)manganese(V) corroles to sulfides. J. Am. Chem. Soc. 132, 15233–15245 (2010).
Lionetti, D. et al. Effects of Lewis acidic metal ions (M) on oxygen-atom transfer reactivity of heterometallic Mn3MO4 cubane and Fe3MO(OH) and Mn3MO(OH) clusters. Inorg. Chem. 58, 2336–2345 (2019).
Paudel, J., Pokhrel, A., Kirk, M. L. & Li, F. Remote charge effects on the oxygen-atom-transfer reactivity and their relationship to molybdenum enzymes. Inorg. Chem. 58, 2054–2068 (2019).
Li, Y. et al. Selective late-stage oxygenation of sulfides with ground-state oxygen by uranyl photocatalysis. Angew. Chem. Int. Ed. 58, 13499–13506 (2019).
Jin, K. et al. Epoxidation of cyclooctene using water as the oxygen atom source at manganese oxide electrocatalysts. J. Am. Chem. Soc. 141, 6413–6418 (2019).
Peariso, K., McNaughton, R. L. & Kirk, M. L. Active-site stereochemical control of oxygen atom transfer reactivity in sulfite oxidase. J. Am. Chem. Soc. 124, 9006–9007 (2002).
McGarrigle, E. M. & Gilheany, D. G. Chromium− and manganese−salen promoted epoxidation of alkenes. Chem. Rev. 105, 1563–1602 (2005).
Avenier, F. et al. Photoassisted generation of a dinuclear iron(III) peroxo species and oxygen-atom transfer. Angew. Chem. Int. Ed. 52, 3634–3637 (2013).
Kang, Y. et al. Mutable properties of nonheme iron(III)–iodosylarene complexes result in the elusive multiple-oxidant mechanism. J. Am. Chem. Soc. 139, 7444–7447 (2017).
Serrano-Plana, J. et al. Exceedingly fast oxygen atom transfer to olefins via a catalytically competent nonheme iron species. Angew. Chem. Int. Ed. 55, 6310–6314 (2016).
Ling, Y., Wang, G., Wheeler, D. A., Zhang, J. Z. & Li, Y. Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano Lett. 11, 2119–2125 (2011).
Goto, Y., Matsui, T., Ozaki, S.-I., Watanabe, Y. & Fukuzumi, S. Mechanisms of sulfoxidation catalyzed by high-valent intermediates of heme enzymes: electron-transfer vs oxygen-transfer mechanism. J. Am. Chem. Soc. 121, 9497–9502 (1999).
Li, Y., Wang, M. & Jiang, X. Controllable sulfoxidation and sulfenylation with organic thiosulfate salts via dual electron- and energy-transfer photocatalysis. ACS Catal. 7, 7587–7592 (2017).
Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007).
Nosaka, Y. & Nosaka, A. Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 117, 11302–11336 (2017).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Badalyan, A. & Stahl, S. S. Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators. Nature 535, 406–410 (2016).
Engelmann, X. et al. Trapping of a highly reactive oxoiron(IV) complex in the catalytic epoxidation of olefins by hydrogen peroxide. Angew. Chem. Int. Ed. 58, 4012–4016 (2019).
Lee, Y. M. et al. Direct oxygen atom transfer versus electron transfer mechanisms in the phosphine oxidation by nonheme Mn(IV)–oxo complexes. Chem. Commun. 53, 9352–9355 (2017).
Man, W.-L., Lam, W. W. Y., Wong, W.-Y. & Lau, T.-C. Oxidation of nitrite by a trans-dioxoruthenium(VI) complex: direct evidence for reversible oxygen atom transfer. J. Am. Chem. Soc. 128, 14669–14675 (2006).
Wang, Z., Bush, R. T., Sullivan, L. A., Chen, C. & Liu, J. Selective oxidation of arsenite by peroxymonosulfate with high utilization efficiency of oxidant. Environ. Sci. Technol. 48, 3978–3985 (2014).
Klahr, B., Gimenez, S., Fabregat-Santiago, F., Hamann, T. & Bisquert, J. Water oxidation at hematite photoelectrodes: the role of surface states. J. Am. Chem. Soc. 134, 4294–4302 (2012).
Le Formal, F. et al. Rate law analysis of water oxidation on a hematite surface. J. Am. Chem. Soc. 137, 6629–6637 (2015).
Mesa, C. A. et al. Multihole water oxidation catalysis on haematite photoanodes revealed by operando spectroelectrochemistry and DFT. Nat. Chem. 12, 82–89 (2020).
Yatom, N., Neufeld, O. & Caspary Toroker, M. Toward settling the debate on the role of Fe2O3 surface states for water splitting. J. Phys. Chem. C 119, 24789–24795 (2015).
Kundu, S., Thompson, J. V., Ryabov, A. D. & Collins, T. J. On the reactivity of mononuclear iron(V)oxo complexes. J. Am. Chem. Soc. 133, 18546–18549 (2011).
Singh, K. K., Tiwari, M. K., Dhar, B. B., Vanka, K. & Gupta, S. S. Mechanism of oxygen atom transfer from FeV(O) to olefins at room temperature. Inorg. Chem. 54, 6112–6121 (2015).
Lim, M. H. et al. An FeIV=O complex of a tetradentate tripodal nonheme ligand. Proc. Natl Acad. Sci. USA 100, 3665–3670 (2003).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Mosey, N. J., Liao, P. & Carter, E. A. Rotationally invariant ab initio evaluation of Coulomb and exchange parameters for DFT+U calculations. J. Chem. Phys. 129, 014103 (2008).
Ji, Y., Fan, T. & Luo, Y. First-principles study on the mechanism of photocatalytic reduction of nitrobenzene on the rutile TiO2(110) surface. Phys. Chem. Chem. Phys. 22, 1187–1193 (2019).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Finger, L. W. & Hazen, R. M. Crystal structure and isothermal compression of Fe2O3, Cr2O3, and V2O3 to 50 kbars. J. Appl. Phys. 51, 5362–5367 (1980).
Hanaor, D. A. H. & Sorrell, C. C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 46, 855–874 (2011).
Wang, V., Xu, N., Liu, J.-C., Tang, G. & Geng, W.-T. VASPKIT: a pre- and post-processing program for VASP code. Cond. Mat. Mater. Sci. 267, 108033 (2021).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23–J26 (2005).
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, grant no. XDB36000000, NSFC (nos. 21777168, 21827809 and 22072158) and the ‘National Key R&D Program of China’ (nos. 2018YFA0209302 and 2020YFC1808401).
Author information
Authors and Affiliations
Contributions
Y. Zhao and C.C. conceived and designed the experiments. Y. Zhao performed most of the experiments. C.D. prepared α-Fe2O3 and contributed the DFT work. D.T. reproduced the results of MPS photoelectrolysis experimental data. L.D. measured and analysed the scanning electron microscopy data. Y. Zhao, C.D., D.T., L.D., Y. Zhang, H.S., H.J., W.S., W.M., C.C. and J.Z. analysed the results and reviewed the paper. C.C., Y. Zhao. and Y. Zhang wrote the paper, with input from the other authors. C.C. directed the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Victor Batista, Alberto Vomiero, Li-Zhu Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Materials and Methods, Figs. 1–29, Tables 1–7 and References.
Supplementary Data 1
Model for Figure S27b(α-Fe2O3).
Supplementary Data 2
Model for Figure S27b(TiO2).
Supplementary Data 3
Model for FeIII-FeIII.
Supplementary Data 4
Model for FeIII-FeIV.
Supplementary Data 5
Model for FeIV-FeIV.
Supplementary Data 6
Model for FeV-FeIII-1.
Supplementary Data 7
Model for FeV-FeIII-2.
Supplementary Data 8
Electronic structure calculations.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Table 1
Statistical Source Data for Table 1.
Rights and permissions
About this article
Cite this article
Zhao, Y., Deng, C., Tang, D. et al. α-Fe2O3 as a versatile and efficient oxygen atom transfer catalyst in combination with H2O as the oxygen source. Nat Catal 4, 684–691 (2021). https://doi.org/10.1038/s41929-021-00659-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00659-1
This article is cited by
-
Stacking textured films on lattice-mismatched transparent conducting oxides via matched Voronoi cell of oxygen sublattice
Nature Materials (2024)
-
Lattice engineered nanoscale Fe0 for selective reductions
Nature Water (2024)
-
Fabrication of novel and promising heterogeneous nanocatalyst Fe2O3-g-C3N4/Cu(II) derived from MIL-53(Fe) and decorated with copper as an efficient and reusable catalyst for Hantzsch reaction
Research on Chemical Intermediates (2024)
-
Approaches to Improving Selectivity During Photoelectrochemical Transformation of Small Molecules
Transactions of Tianjin University (2024)
-
Br−/BrO−-mediated highly efficient photoelectrochemical epoxidation of alkenes on α-Fe2O3
Nature Communications (2023)