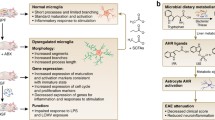
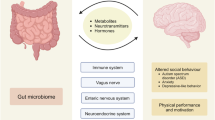
Abstract
The neurodegeneration and its related CNS pathologies need an urgent toolbox to minimize the global mental health burden. The neuroimmune system critically regulates the brain maturation and survival of neurons across the nervous system. The chronic manipulated immunological drive can accelerate the neuronal pathology hence promoting the burden of neurodegenerative disorders. The gut is home for trillions of microorganisms having a mutual relationship with the host system. The gut-brain axis is a unique biochemical pathway through which the gut residing microbes connects with the brain cells and regulates various physiological and pathological cascades. The gut microbiota and CNS communicate using a common language that synchronizes the tuning of immune cells. The intestinal gut microbial community has a profound role in the maturation of the immune system as well as the development of the nervous system. We have critically summarised the clinical and preclinical reports from the past a decade emphasising that the significant changes in gut microbiota can enhance the host susceptibility towards neurodegenerative disorders. In this review, we have discussed how the gut microbiota-mediated immune response inclines the host physiology towards neurodegeneration and indicated the gut microbiota as a potential future candidate for the management of neurodegenerative disorders.



Similar content being viewed by others
References
Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M et al (2016) Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 32:315–320
Amaral FA, Sachs D, Costa VV et al (2008) Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci 105:2193–2197
Appel SH, Zhao W, Beers DR, Henkel JS (2011) The microglial-motoneuron dialogue in ALS. Acta Myol 30:4
Atarashi K, Tanoue T, Ando M et al (2015) Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163:367–380
Bakshi R, Xu Y, Mueller KA et al (2018) Urate mitigates oxidative stress and motor neuron toxicity of astrocytes derived from ALS-linked SOD1G93A mutant mice. Mol Cell Neurosci 92:12–16
Bannister K, Smith RV, Wilkins P, Cummins TM (2021) Towards optimising experimental quantification of persistent pain in Parkinson’s disease using psychophysical testing. npj Park Dis
Bano D, Zanetti F, Mende Y, Nicotera P (2011) Neurodegenerative processes in Huntington’s disease. Cell Death Dis 2:e228–e228
Berer K, Gerdes LA, Cekanaviciute E et al (2017) Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci 114:10719–10724
Berer K, Mues M, Koutrolos M et al (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479:538–541
Bhela S, Kempsell C, Manohar M et al (2015) Nonapoptotic and extracellular activity of granzyme B mediates resistance to regulatory T cell (Treg) suppression by HLA-DR−CD25hiCD127lo Tregs in multiple sclerosis and in response to IL-6. J Immunol 194:2180–2189
Bhuiyan P, Chen Y, Karim M et al (2021) Bidirectional communication between mast cells and the gut-brain axis in neurodegenerative disease: avenues for therapeutic intervention. Brain Res Bull
Binder DK, Scharfman HE (2004) Brain-derived neurotrophic factor. Growth Factors 22:123
Bolte AC, Lukens JR (2018) Th17 cells in Parkinson’s disease: the bane of the midbrain. Cell Stem Cell 23:5–6
Bonfili L, Cecarini V, Berardi S et al (2017) Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep 7:1–21
Braniste V, Al-Asmakh M, Kowal C et al (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263ra158–263ra158
Brenner D, Hiergeist A, Adis C et al (2018) The fecal microbiome of ALS patients. Neurobiol Aging 61:132–137
Breyner NM, Michon C, de Sousa CS et al (2017) Microbial anti-inflammatory molecule (MAM) from Faecalibacterium prausnitzii shows a protective effect on DNBS and DSS-induced colitis model in mice through inhibition of NF-κB pathway. Front Microbiol 8:114
Browne TC, McQuillan K, McManus RM et al (2013) IFN-γ production by amyloid β–specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J Immunol 190:2241–2251
Campos-Acuña J, Elgueta D, Pacheco R (2019) T-cell-driven inflammation as a mediator of the gut-brain axis involved in Parkinson’s disease. Front Immunol 10:239
Cao Y, Goods BA, Raddassi K et al (2015) Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci Transl Med 7:287ra74–287ra74
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol Q Publ Hell Soc Gastroenterol 28:203
Cekanaviciute E, Yoo BB, Runia TF et al (2017) Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci 114:10713–10718
Chang K-H, Wu Y-R, Chen Y-C, Chen C-M (2015) Plasma inflammatory biomarkers for Huntington’s disease patients and mouse model. Brain Behav Immun 44:121–127
Cheng LH, Liu YW, Wu CC et al (2019) Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal
Cheung SG, Goldenthal AR, Uhlemann AC et al (2019) Systematic review of gut microbiota and major depression. Front Psychiatry. https://doi.org/10.3389/fpsyt.2019.00034
Choileáin SN, Kleinewietfeld M, Raddassi K et al (2020) CXCR3+ T cells in multiple sclerosis correlate with reduced diversity of the gut microbiome. J Transl Autoimmun. https://doi.org/10.1016/j.jtauto.2019.100032
Chunchai T, Thunapong W, Yasom S et al (2018) Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J Neuroinflammation 15:1–15
Clarke G, O’Mahony SM, Dinan TG, Cryan JF (2014) Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr 103:812–819
Costa MC, Santos JRA, Ribeiro MJA et al (2016) The absence of microbiota delays the inflammatory response to Cryptococcus gattii. Int J Med Microbiol 306:187–195
Cryan JF, Dinan TG (2015) Gut microbiota: microbiota and neuroimmune signalling—Metchnikoff to microglia. Nat Rev Gastroenterol Hepatol 12:494–496
Dahlin M, Prast-Nielsen S (2019) The gut microbiome and epilepsy. EBioMedicine 44:741–746. https://doi.org/10.1016/j.ebiom.2019.05.024
Danikowski KM, Jayaraman S, Prabhakar B (2017) Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation 14:117
De Paola M, Sestito SE, Mariani A et al (2016) Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model. Pharmacol Res 103:180–187
Dinan TG, Cryan JF (2017) Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol 595:489–503
Donaldson DS, Bradford BM, Artis D, Mabbott NA (2015) Reciprocal regulation of lymphoid tissue development in the large intestine by IL-25 and IL-23. Mucosal Immunol 8:582–595
Du G, Dong W, Yang Q et al (2021) Altered gut microbiota related to inflammatory responses in patients with Huntington’s disease. Front Immunol. https://doi.org/10.3389/fimmu.2020.603594
Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK et al (2018) Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. https://doi.org/10.1016/j.neuron.2018.06.030
Ellrichmann G, Reick C, Saft C, Linker RA (2013) The role of the immune system in Huntington’s disease. Clin Dev Immunol 2013
Engen PA, Zaferiou A, Rasmussen H et al (2020) Single-arm, non-randomized, time series, single-subject study of fecal microbiota transplantation in multiple sclerosis. Front Neurol. https://doi.org/10.3389/fneur.2020.00978
Erny D, de Angelis ALH, Jaitin D et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18:965
Fan Y, Wang H, Liu X et al (2019) Crosstalk between the ketogenic diet and epilepsy: from the perspective of gut microbiota. Mediators Inflamm. https://doi.org/10.1155/2019/8373060
Fang X (2016) Potential role of gut microbiota and tissue barriers in Parkinson’s disease and amyotrophic lateral sclerosis. Int J Neurosci 126:771–776
Farooqui AA (2021) Contribution of gut microbiota in the pathogenesis of amyotrophic lateral sclerosis and Huntington’s disease. In: Gut microbiota in neurologic and visceral diseases
Ferretti P, Pasolli E, Tett A et al (2018) Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24:133–145
Foster JA, Neufeld K-AM (2013) Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–312
Francino MP (2018) Birth mode-related differences in gut microbiota colonization and immune system development. Ann Nutr Metab 73:12–16
Fülling C, Lach G, Bastiaanssen TFS et al (2020) Adolescent dietary manipulations differentially affect gut microbiota composition and amygdala neuroimmune gene expression in male mice in adulthood. Brain Behav Immun 87:666–678
Fung TC (2020) The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis 136:104714
Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20:145
Gabriel FC, Fantuzzi G (2019) The association of short-chain fatty acids and leptin metabolism: a systematic review. Nutr Res
Gorecki AM, Preskey L, Bakeberg MC et al (2019) Altered gut microbiome in Parkinson’s disease and the influence of lipopolysaccharide in a human α-synuclein over-expressing mouse model. Front Neurosci. https://doi.org/10.3389/fnins.2019.00839
Gross R (2017) Is cesarean section associated with risk for autism spectrum disorder? Eur Neuropsychopharmacol 27:S749
Gualtieri P, Marchetti M, Cioccoloni G et al (2020) Psychobiotics regulate the anxiety symptoms in carriers of allele A of IL-1 β gene: a randomized, placebo-controlled clinical trial. Mediators Inflamm. https://doi.org/10.1155/2020/2346126
Gutzeit C, Magri G, Cerutti A (2014) Intestinal IgA production and its role in host-microbe interaction. Immunol Rev 260:76–85
Hang Mak CC, Hong Meng HY, Yan Mak JW, et al (2020) IDDF2020-ABS-0203 Investigating the evidence of prebiotic supplementation in the attenuation of age-related neurodegeneration in in vivo studies: a systematic review and meta-analysis with bayesian inference
Hazan S (2020) Rapid improvement in Alzheimer’s disease symptoms following fecal microbiota transplantation: a case report. J Int Med Res 48:0300060520925930. https://doi.org/10.1177/0300060520925930
Heijtz RD, Wang S, Anuar F et al (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci 108:3047–3052
Henry RJ, Ritzel RM, Barrett JP et al (2020) Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci 40:2960–2974
Hertzberg VS, Singh H, Fournier CN et al (2021) Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph Lateral Scler Front Degener. https://doi.org/10.1080/21678421.2021.1904994
Hrncir T, Stepankova R, Kozakova H et al (2008) Gut microbiota and lipopolysaccharide content of the diet influence development of regulatory T cells: studies in germ-free mice. BMC Immunol 9:65
Hsieh-Li HM, Lee GC, Lee YS et al (2021) Prebiotic lactulose ameliorates the cognitive deficit in Alzheimer’s disease mouse model through macroautophagy and chaperone-mediated autophagy pathways. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.0c07327
Iannone LF, Gómez-Eguílaz M, Citaro R, Russo E (2020) The potential role of interventions impacting on gut-microbiota in epilepsy. Exp Rev Clin Pharmacol 13:423–435. https://doi.org/10.1080/17512433.2020.1759414
Ikawa M, Okazawa H, Tsujikawa T et al (2015) Increased oxidative stress is related to disease severity in the ALS motor cortex: a PET study. Neurology 84:2033–2039
Jameson KG, Hsiao EY (2018) Linking the gut microbiota to a brain neurotransmitter. Trends Neurosci 41:413–414
Janik R, Thomason LAM, Stanisz AM et al (2016) Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 125:988–995
Kabouridis PS, Lasrado R, McCallum S et al (2015) The gut microbiota keeps enteric glial cells on the move; prospective roles of the gut epithelium and immune system. Gut Microbes. https://doi.org/10.1080/19490976.2015.1109767
Kadowaki A, Saga R, Lin Y et al (2019) Gut microbiota-dependent CCR9+ CD4+ T cells are altered in secondary progressive multiple sclerosis. Brain. https://doi.org/10.1093/brain/awz012
Kang D-W, Park JG, Ilhan ZE, et al (2013) Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 8
Keshavarzian A, Green SJ, Engen PA et al (2015) Colonic bacterial composition in Parkinson’s disease. Mov Disord 30:1351–1360
Kim CH, Park J, Kim M (2014) Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw 14:277–288
Kong G, Lê Cao K-A, Judd LM et al (2018a) Microbiome profiling reveals gut dysbiosis in a transgenic mouse model of Huntington’s disease. Neurobiol Dis 104268
Kong H, Dong C, Tian Z et al (2018b) Altered immunity in crowded Mythimna separata is mediated by octopamine and dopamine. Sci Rep 8:1–10
Krajmalnik-Brown R, Kang D-W, Park JG et al (2017) Microbiome markers and therapies for autism spectrum disorders
Kuai X, Yao X, Xu L et al (2021) Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb Cell Fact 20:98. https://doi.org/10.1186/s12934-021-01589-0
Kurokawa S, Kishimoto T, Mizuno S et al (2018) The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord 235:506–512
Kustrimovic N, Comi C, Magistrelli L et al (2018) Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: Cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J Neuroinflammation. https://doi.org/10.1186/s12974-018-1248-8
Langen UH, Ayloo S, Gu C (2019) Development and cell biology of the blood-brain barrier. Annu Rev Cell Dev Biol 35:591–613
Lass-Flörl C, Dierich MP, Fuchs D et al (2001) Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline. Clin Infect Dis 33:e135–e136
Lee KE, Kim JK, Kim DH (2020) Orally administered antibiotics vancomycin and ampicillin cause cognitive impairment with gut dysbiosis in mice with transient global forebrain ischemia. Front Microbiol. https://doi.org/10.3389/fmicb.2020.564271
Lee SJ, Sim G-Y, Lee Y et al (2017) Engineering of Escherichia coli for the synthesis of N-hydroxycinnamoyl tryptamine and serotonin. J Ind Microbiol Biotechnol 44:1551–1560
Lew L-C, Hor Y-Y, Yusoff NA et al (2019) Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin Nutr 38:2053–2064
Li Z, Zhu H, Guo Y et al (2020) Gut microbiota regulate cognitive deficits and amyloid deposition in a model of Alzheimer’s disease. J Neurochem. https://doi.org/10.1111/jnc.15031
Liang S, Wu X, Hu X et al (2018) Recognizing depression from the microbiota–gut–brain axis. Int J Mol Sci. https://doi.org/10.3390/ijms19061592
Liao JF, Cheng YF, You ST et al (2020) Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models of Parkinson’s disease. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.07.036
Lin C-H, Chen C-C, Chiang H-L et al (2019) Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation 16:1–9
Lin S, Mukherjee S, Li J et al (2021) Mucosal immunity–mediated modulation of the gut microbiome by oral delivery of probiotics into Peyer’s patches. Sci Adv 7:eabf0677
Liu G, Zhao Y (2007) Toll-like receptors and immune regulation: their direct and indirect modulation on regulatory CD4+ CD25+ T cells. Immunology 122:149–156
Liu S, Gao J, Zhu M et al (2020) Gut microbiota and dysbiosis in alzheimer’s disease: implications for pathogenesis and treatment. Mol. Neurobiol
Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12:661–672
Ma Q, Xing C, Long W et al (2019a) Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation 16:1–14
Ma Q, Xing C, Long W et al (2019b) Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J. Neuroinflammation
Macrì S, Spinello C, Widomska J et al (2018) Neonatal corticosterone mitigates autoimmune neuropsychiatric disorders associated with streptococcus in mice. Sci Rep 8:1–12
Magistrelli L, Storelli E, Rasini E et al (2020) Relationship between circulating CD4+ T lymphocytes and cognitive impairment in patients with Parkinson’s disease. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.07.005
Magna M, Pisetsky DS (2014) The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med 20:138–146
Maher SE, O’Brien EC, Moore RL, et al (2020) The association between the maternal diet and the maternal and infant gut microbiome: a systematic review. Br J Nutr 1–29
Makkawi S, Camara-Lemarroy C, Metz L (2018) Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol NeuroInflamm. https://doi.org/10.1212/NXI.0000000000000459
Mandrioli J, Amedei A, Cammarota G et al (2019) FETR-ALS study protocol: a randomized clinical trial of fecal microbiota transplantation in amyotrophic lateral sclerosis. Front Neurol. https://doi.org/10.3389/fneur.2019.01021
Maslowski KM, Mackay CR (2011) Diet, gut microbiota and immune responses. Nat Immunol 12:5–9
Mayer EA, Tillisch K, Gupta A (2015) Gut/brain axis and the microbiota. J Clin Invest 125:926–938
Mazzini L, Mogna L, De Marchi F et al (2018) Potential role of gut microbiota in ALS pathogenesis and possible novel therapeutic strategies. J Clin Gastroenterol 52:S68–S70
Mertsalmi TH, Aho VTE, Pereira PAB et al (2017) More than constipation–bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur J Neurol 24:1375–1383
Messaoudi M, Violle N, Bisson J-F et al (2011) Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2:256–261
Mezö C, Dokalis N, Mossad O et al (2020) Different effects of constitutive and induced microbiota modulation on microglia in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun. https://doi.org/10.1186/s40478-020-00988-5
Mittal K, Eremenko E, Berner O et al (2019) CD4 T cells induce a subset of MHCII-expressing microglia that attenuates Alzheimer pathology. Iscience 16:298–311
Miyake S, Kim S, Suda W, et al (2015) Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS ONE 10
Moore RE, Townsend SD (2019) Temporal development of the infant gut microbiome. Open Biol 9:190128
Morais LH, Hara DB, Bicca MA et al (2018) Early signs of colonic inflammation, intestinal dysfunction, and olfactory impairments in the rotenone-induced mouse model of Parkinson’s disease. Behav Pharmacol 29:199–210
Morikawa M, Tsujibe S, Kiyoshima-Shibata J et al (2016) Microbiota of the small intestine is selectively engulfed by phagocytes of the lamina propria and Peyer’s patches. PLoS ONE 11
Mörkl S, Butler MI, Holl A et al (2020) Probiotics and the microbiota-gut-brain axis: focus on psychiatry. Curr Nutr Rep 9:171–182. https://doi.org/10.1007/s13668-020-00313-5
Moschopoulos C, Kratimenos P, Koutroulis I et al (2018) The neurodevelopmental perspective of surgical necrotizing enterocolitis: the role of the gut-brain axis. Mediators Inflamm 2018
Mosher KI, Wyss-Coray T (2015) Go with your gut: microbiota meet microglia. Nat Neurosci 18:930–931
Nagai M, Re DB, Nagata T et al (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci 10:615–622
Nicholson JK, Holmes E, Kinross J et al (2012) Host-gut microbiota metabolic interactions. Science (80-) 336:1262–1267
Nuzum ND, Loughman A, Szymlek-Gay EA et al (2020) Gut microbiota differences between healthy older adults and individuals with Parkinson’s disease: a systematic review. Neurosci Biobehav Rev
Nzakizwanayo J, Dedi C, Standen G et al (2015) Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci Rep 5:17324
O’Donovan SM, Crowley EK, Brown JRM et al (2020) Nigral overexpression of α-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol Motil. https://doi.org/10.1111/nmo.13726
Olson CA, Vuong HE, Yano JM et al (2018) The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173:1728–1741. https://doi.org/10.1016/j.cell.2018.04.027
Pan J-X, Deng F-L, Zeng B-H et al (2019) Absence of gut microbiota during early life affects anxiolytic Behaviors and monoamine neurotransmitters system in the hippocampal of mice. J Neurol Sci 400:160–168
Paulsen JS, Nopoulos PC, Aylward E et al (2010) Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain Res Bull 82:201–207
Pearson-Leary J, Zhao C, Bittinger K et al (2019) The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry 1–12
Pellegrini C, Antonioli L, Calderone V et al (2020) Microbiota-gut-brain axis in health and disease: Is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications? Prog Neurobiol 191:101806
Pellegrini C, Antonioli L, Colucci R et al (2018) Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol 136:345–361
Philips T, Robberecht W (2011) Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol 10:253–263
Pokusaeva K, Johnson C, Luk B et al (2017) GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil 29:e12904
Powell N, Walker MM, Talley NJ (2017) The mucosal immune system: master regulator of bidirectional gut–brain communications. Nat Rev Gastroenterol Hepatol 14:143
Pröbstel AK, Zhou X, Baumann R et al (2020) Gut microbiota–specific iga+ B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. https://doi.org/10.1126/SCIIMMUNOL.ABC7191
Puentes F, Malaspina A, Van Noort JM, Amor S (2016) Non-neuronal cells in ALS: role of glial, immune cells and blood-CNS barriers. Brain Pathol 26:248–257
Qian K, Huang H, Peterson A et al (2017) Sporadic ALS astrocytes induce neuronal degeneration in vivo. Stem Cell Reports 8:843–855
Quévrain E, Maubert MA, Michon C et al (2016) Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 65:415–425
Radulescu CI, Garcia-Miralles M, Sidik H, et al (2018) Manipulation of microbiota reveals altered myelination and white matter plasticity in a model of Huntington disease. bioRxiv
Radulescu CI, Garcia-Miralles M, Sidik H et al (2020) Reprint of: Manipulation of microbiota reveals altered callosal myelination and white matter plasticity in a model of Huntington disease. Neurobiol Dis. https://doi.org/10.1016/j.nbd.2020.104744
Reiff C, Kelly D (2010) Inflammatory bowel disease, gut bacteria and probiotic therapy. Int J Med Microbiol 300:25–33
Rhee SH, Pothoulakis C, Mayer EA (2009) Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6:306
Ribeiro SC, Domingos-Lopes MFP, Stanton C et al (2018) Production of-aminobutyric acid (GABA) by Lactobacillus otakiensis and other Lactobacillus sp. isolated from traditional Pico cheese. Int J Dairy Technol 71:1012–1017
Rosenblum LT, Shamamandri-Markandaiah S, Ghosh B et al (2017) Mutation of the caspase-3 cleavage site in the astroglial glutamate transporter EAAT2 delays disease progression and extends lifespan in the SOD1-G93A mouse model of ALS. Exp Neurol 292:145–153
Round JL, Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9:313–323
Saksida T, Koprivica I, Vujičić M et al (2017) Impaired IL-17 production in gut-residing immune cells of 5xFAD mice with alzheimer’s disease pathology. J Alzheimer’s Dis. https://doi.org/10.3233/JAD-170538
Sampson TR, Debelius JW, Thron T et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167:1469–1480
Saraf MK, Piccolo BD, Bowlin AK et al (2017) Formula diet driven microbiota shifts tryptophan metabolism from serotonin to tryptamine in neonatal porcine colon. Microbiome 5:77
Saresella M, Marventano I, Barone M et al (2020) Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Front Immunol. https://doi.org/10.3389/fimmu.2020.01390
Sawada M, Imamura K, Nagatsu T (2006) Role of cytokines in inflammatory process in Parkinson’s disease. In: Parkinson’s disease and related disorders. Springer, pp 373–381
Schwiertz A, Spiegel J, Dillmann U et al (2018) Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord 50:104–107
Scott GA, Terstege DJ, Vu AP et al (2020) Disrupted neurogenesis in germ-free mice: effects of age and sex. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2020.00407
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608
Shen H, Guan Q, Zhang X et al (2020) New mechanism of neuroinflammation in Alzheimer’s disease: the activation of NLRP3 inflammasome mediated by gut microbiota. Prog Neuro-Psychopharmacol Biol Psychiatry. https://doi.org/10.1016/j.pnpbp.2020.109884
Sherman MP, Zaghouani H, Niklas V (2015) Gut microbiota, the immune system, and diet influence the neonatal gut–brain axis. Pediatr Res 77:127–135
Shi H, Wang Q, Zheng M et al (2020) Supplement of microbiota-accessible carbohydrates prevents neuroinflammation and cognitive decline by improving the gut microbiota-brain axis in diet-induced obese mice. J Neuroinflammation. https://doi.org/10.1186/s12974-020-01760-1
Shmuel-Galia L, Klug Y, Porat Z et al (2017) Intramembrane attenuation of the TLR4-TLR6 dimer impairs receptor assembly and reduces microglia-mediated neurodegeneration. J Biol Chem 292:13415–13427
Silva RAC, da Silva CR, de Andrade Neto JB et al (2017) In vitro anti-Candida activity of selective serotonin reuptake inhibitors against fluconazole-resistant strains and their activity against biofilm-forming isolates. Microb Pathog 107:341–348
Simpson DSA, Oliver PL (2020) ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 9:743
Smith PM, Howitt MR, Panikov N et al (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science (80-) 341:569–573
Sokoloff B, Saelhof CC, Yoshino A (1967) Candida albicans as a producer of serotonin. Growth 31:297–300
Sommer A, Marxreiter F, Krach F et al (2018) Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson’s disease. Cell Stem Cell 23:123–131
Sorce S, Stocker R, Seredenina T et al (2017) NADPH oxidases as drug targets and biomarkers in neurodegenerative diseases: what is the evidence? Free Radic Biol Med 112:387–396
Spiljar M, Merkler D, Trajkovski M (2017) The immune system bridges the gut microbiota with systemic energy homeostasis: focus on TLRs, mucosal barrier, and SCFAs. Front Immunol 8:1353
Srivastav S, Neupane S, Bhurtel S et al (2019) Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J Nutr Biochem. https://doi.org/10.1016/j.jnutbio.2019.03.021
Stanisavljević S, Čepić A, Bojić S et al (2019) Oral neonatal antibiotic treatment perturbs gut microbiota and aggravates central nervous system autoimmunity in Dark Agouti rats. Sci Rep 9:1–13
Sun J, Xu J, Ling Y et al (2019) Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. https://doi.org/10.1038/s41398-019-0525-3
Sun J, Xu J, Yang B et al (2020) Effect of clostridium butyricum against microglia-mediated neuroinflammation in Alzheimer’s disease via regulating gut microbiota and metabolites butyrate. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201900636
Sun L, Shen R, Agnihotri SK et al (2018a) Lack of PINK1 alters glia innate immune responses and enhances inflammation-induced, nitric oxide-mediated neuron death. Sci Rep 8:1–16
Sun M-F, Shen Y-Q (2018) Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res Rev 45:53–61
Sun MF, Zhu YL, Zhou ZL et al (2018b) Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2018.02.005
Thompson AM, Bizzarro MJ (2008) Necrotizing enterocolitis in newborns. Drugs 68:1227–1238
Tian T, Xu B, Qin Y et al (2019) Clostridium butyricum miyairi 588 has preventive effects on chronic social defeat stress-induced depressive-like behaviour and modulates microglial activation in mice. Biochem Biophys Res Commun 516:430–436
Tlaskalová-Hogenová H, Štěpánková R, Kozáková H et al (2011) The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol 8:110
Tsunoda I (2017) Lymphatic system and gut microbiota affect immunopathology of neuroinflammatory diseases, including multiple sclerosis, neuromyelitis optica and Alzheimer’s disease. Clin Exp Neuroimmunol
Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A et al (2014) Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 30:430–435
Valles-Colomer M, Falony G, Darzi Y et al (2019) The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 4:623–632. https://doi.org/10.1038/s41564-018-0337-x
van Pamelen J, van Olst L, Budding AE et al (2020) Alterations of gut microbiota and the brain-immune-intestine axis in patients with relapsing-remitting multiple sclerosis after treatment with oral cladribine: protocol for a prospective observational study. JMIR Res Protoc. https://doi.org/10.2196/16162
van Thiel IAM, De Jonge WJ, Chiu IM, van den Wijngaard RM (2020) Microbiota-neuroimmune cross talk in stress-induced visceral hypersensitivity of the bowel. Am J Physiol Liver Physiol 318:G1034–G1041
Vascellari S, Melis M, Palmas V et al (2021) Clinical phenotypes of Parkinson’s disease associate with distinct gut microbiota and metabolome enterotypes. Biomolecules. https://doi.org/10.3390/biom11020144
Vrieze A, Holleman F, Zoetendal EG et al (2010) The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia 53:606–613
Vulevic J, Juric A, Walton GE et al (2015) Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr 114:586–595
Wang L, Fleming SM, Chesselet M-F, Taché Y (2008) Abnormal colonic motility in mice overexpressing human wild-type α-synuclein. NeuroReport 19:873
Wassenaar TM, Panigrahi P (2014) Is a foetus developing in a sterile environment? Lett Appl Microbiol 59:572–579
Whitehead WE, Crowell MD, Robinson JC et al (1992) Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut 33:825–830
Wingo AP, Liu Y, Gerasimov ES et al (2021) Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nat Genet. https://doi.org/10.1038/s41588-020-00773-z
Wrzosek L, Miquel S, Noordine M-L et al (2013) Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11:61
Wu S, Yi J, Zhang YG et al (2015) Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep. https://doi.org/10.14814/phy2.12356
Wu Y, Liu W, Li Q et al (2018) Dietary chlorogenic acid regulates gut microbiota, serum-free amino acids and colonic serotonin levels in growing pigs. Int J Food Sci Nutr 69:566–573
Xiang P, Chew WS, Seow WL et al (2021) The S1P2 receptor regulates blood-brain barrier integrity and leukocyte extravasation with implications for neurodegenerative disease. Neurochem Int 146:105018
Ximenez C, Torres J (2017) Development of microbiota in infants and its role in maturation of gut mucosa and immune system. Arch Med Res 48:666–680
Xu N, Fan W, Zhou X et al (2018) Probiotics decrease depressive behaviors induced by constipation via activating the AKT signaling pathway. Metab Brain Dis 33:1625–1633
Xu R, Wu B, Liang J et al (2020) Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav Immun 85:120–127. https://doi.org/10.1016/j.bbi.2019.06.039
Xue LJ, Yang XZ, Tong Q et al (2020) Fecal microbiota transplantation therapy for Parkinson’s disease: a preliminary study. Medicine (baltimore). https://doi.org/10.1097/MD.0000000000022035
Yan FF, Wang WC, Cheng HW (2018) Bacillus subtilis based probiotic improved bone mass and altered brain serotoninergic and dopaminergic systems in broiler chickens. J Funct Foods 49:501–509
Yang J, Zhao Y, Zhang L et al (2018) RIPK3/MLKL-mediated neuronal necroptosis modulates the M1/M2 polarization of microglia/macrophages in the ischemic cortex. Cereb Cortex 28:2622–2635
Yu W-C, Cong J-P, Mi L-Y (2018) Expressions of TOLL-like receptor 4 (TLR-4) and matrix metalloproteinase 9 (MMP-9)/Tissue inhibitor of metalloproteinase 1 (TIMP-1) in pulmonary blood vessels with chronic obstructive pulmonary diseases and their relationships with pulmonary vascular remo. Rev Assoc Med Bras 64:361–367
Yuan X, Kang Y, Zhuo C, Huang X (2019) Biochemical and biophysical research communications the gut microbiota promotes the pathogenesis of schizophrenia via multiple pathways. Biochem Biophys Res Commun 512:373–380. https://doi.org/10.1016/j.bbrc.2019.02.152
Yunes RA, Poluektova EU, Dyachkova MS et al (2016) GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 42:197–204
Zhang C, Wang H, Chen T (2019) Interactions between intestinal microflora/probiotics and the immune system. Biomed Res Int 2019
Zhang YG, Wu S, Yi J et al (2017) Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin Ther. https://doi.org/10.1016/j.clinthera.2016.12.014
Zhang Z, Li J, Zheng W et al (2016) Peripheral lymphoid volume expansion and maintenance are controlled by gut microbiota via RALDH+ dendritic cells. Immunity 44:330–342
Zhao D, Feng F, Zhao C et al (2018) Role of perforin secretion from CD8+ T-cells in neuronal cytotoxicity in multiple sclerosis. Neurol Res 40:62–67
Zhou J, He F, Yang F et al (2018) Increased stool immunoglobulin A level in children with autism spectrum disorders. Res Dev Disabil 82:90–94. https://doi.org/10.1016/j.ridd.2017.10.009
Zhuang ZQ, Shen LL, Li WW et al (2018) Gut microbiota is altered in patients with alzheimer’s disease. J Alzheimer’s Dis. https://doi.org/10.3233/JAD-180176
Acknowledgements
We would like to acknowledge the Department of Pharmaceutical Engineering and Technology, Indian Institute of Technology, Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India for providing the necessary facilities and infrastructure.
Funding
This work is supported by the SPARC grant (SPARC/2018-2019/P435/SL) and Core Research Grant (CRG/2020/002621/BHS) awarded to Dr. Vinod Tiwari by Ministry of Human Resource & Development, Government of India and Science and Engineering Research Board respectively. This work is also supported by Senior Research Fellowship awarded to Mr. Ankit Uniyal under the supervision of Dr. Vinod Tiwari by Indian Council of Medical Research (5/3/8/44/ITR-F/2019-ITR).
Author information
Authors and Affiliations
Contributions
AU and VT conceived the idea and performed the literature review. AU, VT and MR has written the first draft of the manuscript. Further, AU and VT performed critical editing and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uniyal, A., Tiwari, V., Rani, M. et al. Immune-microbiome interplay and its implications in neurodegenerative disorders. Metab Brain Dis 37, 17–37 (2022). https://doi.org/10.1007/s11011-021-00807-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00807-3