Abstract
Parkinson’s disease (PD) ranks first in the world as a neurodegenerative movement disorder and occurs most commonly in an idiopathic form. PD patients may have motor symptoms, non-motor symptoms, including cognitive and behavioral changes, and symptoms related to autonomic nervous system (ANS) failures, such as gastrointestinal, urinary, and cardiovascular symptoms. Unfortunately, the diagnostic accuracy of PD by general neurologists is relatively low. Currently, there is no objective molecular or biochemical test for PD; its diagnosis is based on clinical criteria, mainly by cardinal motor symptoms, which manifest when patients have lost about 60–80% of dopaminergic neurons. Therefore, it is urgent to establish a panel of biomarkers for the early and accurate diagnosis of PD. Once the disease is accurately diagnosed, it may be easier to unravel idiopathic PD’s pathogenesis, and ultimately, finding a cure. This review discusses several biomarkers’ potential to set a panel for early idiopathic PD diagnosis and future directions.
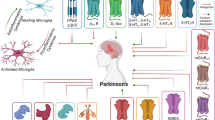
Graphical abstract



Similar content being viewed by others
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP (2008) Epidemiology of Parkinson’s disease. J Neurol 255(Suppl 5):18–32. https://doi.org/10.1007/s00415-008-5004-3
Horowitz MP, Greenamyre JT (2010) Gene-environment interactions in Parkinson’s disease: the importance of animal modeling. Clin Pharmacol Ther 88(4):467–474. https://doi.org/10.1038/clpt.2010.138[pii]
Allam MF, Del Castillo AS, Navajas RF-C (2005) Parkinson’s disease risk factors: genetic, environmental, or both? Neurol Res 27(2):206–208. https://doi.org/10.1179/016164105X22057
Hornykiewicz O (2006) The discovery of dopamine deficiency in the parkinsonian brain. J Neural Transm Suppl 70:9–15. https://doi.org/10.1007/978-3-211-45295-0_3
Shults CW (2006) Lewy bodies. Proc Natl Acad Sci U S A 103(6):1661–1668. https://doi.org/10.1073/pnas.0509567103
Levy OA, Malagelada C, Greene LA (2009) Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis 14(4):478–500. https://doi.org/10.1007/s10495-008-0309-3
Beitz JM (2014) Parkinson’s disease: a review. Front Biosci (Schol Ed) 6:65–74. https://doi.org/10.2741/s415
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39. https://doi.org/10.1001/archneur.56.1.33
Löhle M, Storch A, Reichmann H (2009) Beyond tremor and rigidity: non-motor features of Parkinson’s disease. J Neural Transm (Vienna) 116(11):1483–1492. https://doi.org/10.1007/s00702-009-0274-1
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, et al (2001) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57(3):456–462. https://doi.org/10.1212/wnl.57.3.456
Hornykiewicz O (2002) Dopamine miracle: from brain homogenate to dopamine replacement. Mov Disord 17(3):501–508. https://doi.org/10.1002/mds.10115
Rao SS, Hofmann LA, Shakil A (2006) Parkinson’s disease: diagnosis and treatment. Am Fam Physician 74(12):2046–2054
Duarte-Jurado AP, Gopar-Cuevas Y, Saucedo-Cardenas O, Loera-Arias MJ, Montes-de-Oca-Luna R, Garcia-Garcia A, Rodriguez-Rocha H (2021) Antioxidant therapeutics in Parkinson’s disease: current challenges and opportunities. Antioxidants 10(3):453. https://doi.org/10.3390/antiox10030453
Stoker TB, Barker RA (2020) Recent developments in the treatment of Parkinson’s disease. F1000Res 9:862. https://doi.org/10.12688/f1000research.25634.1
Balestrino R, Schapira AHV (2020) Parkinson disease. Eur J Neurol 27(1):27–42. https://doi.org/10.1111/ene.14108
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. https://doi.org/10.1136/jnnp.2007.131045
Wu Y, Yao Q, Jiang GX, Wang G, Cheng Q (2020) Identification of distinct blood-based biomarkers in early stage of Parkinson’s disease. Neurol Sci 41(4):893–901. https://doi.org/10.1007/s10072-019-04165-y
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, et al (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170. https://doi.org/10.1002/mds.22340
Chaudhuri KR, Schrag A, Weintraub D, Rizos A, Rodriguez-Blazquez C, Mamikonyan E, Martinez-Martin P (2020) The movement disorder society nonmotor rating scale: initial validation study. Mov Disord 35(1):116–133. https://doi.org/10.1002/mds.27862
Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB, Disease MTFotDoPs (2019) Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord 34(10):1464–1470. https://doi.org/10.1002/mds.27802
Marsili L, Rizzo G, Colosimo C (2018) Diagnostic criteria for Parkinson’s disease: from James Parkinson to the concept of prodromal disease. Front Neurol 9:156. https://doi.org/10.3389/fneur.2018.00156
Joutsa J, Gardberg M, Röyttä M, Kaasinen V (2014) Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism Relat Disord 20(8):840–844. https://doi.org/10.1016/j.parkreldis.2014.04.019
Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86(6):566–576. https://doi.org/10.1212/WNL.0000000000002350
Kalender WA (2006) X-ray computed tomography. Phys Med Biol 51(13):R29-43. https://doi.org/10.1088/0031-9155/51/13/R03
Emamzadeh FN, Surguchov A (2018) Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci 12:612. https://doi.org/10.3389/fnins.2018.00612
Lotankar S, Prabhavalkar KS, Bhatt LK (2017) Biomarkers for Parkinson’s disease: recent advancement. Neurosci Bull 33(5):585–597. https://doi.org/10.1007/s12264-017-0183-5
Meyer PT, Frings L, Rücker G, Hellwig S (2017) F-FDG PET in Parkinsonism: differential diagnosis and evaluation of cognitive impairment. J Nucl Med 58(12):1888–1898. https://doi.org/10.2967/jnumed.116.186403
Skowronek C, Zange L, Lipp A (2019) Cardiac 123I-MIBG scintigraphy in neurodegenerative Parkinson syndromes: performance and pitfalls in clinical practice. Front Neurol 10:152. https://doi.org/10.3389/fneur.2019.00152
Rascol O, Schelosky L (2009) 123I-metaiodobenzylguanidine scintigraphy in Parkinson’s disease and related disorders. Mov Disord 24(Suppl 2):S732–741. https://doi.org/10.1002/mds.22499
Group BDW (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69(3):89–95 https://doi.org/10.1067/mcp.2001.113989
Huss R (2015) Biomarkers. In: Atala A, Allickson JG (eds) Translational regenerative medicine. pp 235–241. https://doi.org/10.1016/b978-0-12-410396-2.00019-0
Strimbu K, Tavel JA (2010) What are biomarkers? Curr Opin HIV AIDS 5(6):463–466. https://doi.org/10.1097/COH.0b013e32833ed177
Mayeux R (2004) Biomarkers: potential uses and limitations. NeuroRx 1(2):182–188. https://doi.org/10.1602/neurorx.1.2.182
Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, Mollenhauer B, Blennow K, et al (2019) CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 18(6):573–586. https://doi.org/10.1016/S1474-4422(19)30024-9
Espay AJ, Schwarzschild MA, Tanner CM, Fernandez HH, Simon DK, Leverenz JB, Merola A, Chen-Plotkin A, et al (2017) Biomarker-driven phenotyping in Parkinson’s disease: a translational missing link in disease-modifying clinical trials. Mov Disord 32(3):319–324. https://doi.org/10.1002/mds.26913
Breydo L, Wu JW (1822) Uversky VN (2012) Α-synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta 2:261–285. https://doi.org/10.1016/j.bbadis.2011.10.002
Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M (2005) Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc Natl Acad Sci U S A 102(5):1430–1435. https://doi.org/10.1073/pnas.0407146102
Villar-Piqué A, Lopes da Fonseca T, Outeiro TF (2016) Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies. J Neurochem 139(Suppl 1):240–255. https://doi.org/10.1111/jnc.13249
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, et al (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302(5646):841. https://doi.org/10.1126/science.1090278
Smith WW, Margolis RL, Li X, Troncoso JC, Lee MK, Dawson VL, Dawson TM, Iwatsubo T, et al (2005) Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J Neurosci 25(23):5544–5552. https://doi.org/10.1523/JNEUROSCI.0482-05.2005
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, et al (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4(2):160–164. https://doi.org/10.1038/ncb748
Chahine LM, Beach TG, Brumm MC, Adler CH, Coffey CS, Mosovsky S, Caspell-Garcia C, Serrano GE, et al (2020) In vivo distribution of α-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology 95(9):e1267–e1284. https://doi.org/10.1212/WNL.0000000000010404
Castanedo-Cazares JP, Rodriguez-Leyva I (2015) Skin biomarkers for neurodegenerative disease: a future perspective. Neurodegener Dis Manag 5(6):465–467. https://doi.org/10.2217/nmt.15.51
Teves JMY, Bhargava V, Kirwan KR, Corenblum MJ, Justiniano R, Wondrak GT, Anandhan A, Flores AJ, et al (2017) Parkinson’s disease skin fibroblasts display signature alterations in growth, redox homeostasis, mitochondrial function, and autophagy. Front Neurosci 11:737. https://doi.org/10.3389/fnins.2017.00737
Rodríguez-Leyva I, Calderón-Garcidueñas AL, Jiménez-Capdeville ME, Rentería-Palomo AA, Hernandez-Rodriguez HG, Valdés-Rodríguez R, Fuentes-Ahumada C, Torres-Álvarez B, et al (2014) α-Synuclein inclusions in the skin of Parkinson’s disease and parkinsonism. Ann Clin Transl Neurol 1(7):471–478. https://doi.org/10.1002/acn3.78
Atarashi R, Sano K, Satoh K, Nishida N (2011) Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion 5(3):150–153. https://doi.org/10.4161/pri.5.3.16893
Barria MA, Gonzalez-Romero D, Soto C (2012) Cyclic amplification of prion protein misfolding. Methods Mol Biol 849:199–212. https://doi.org/10.1007/978-1-61779-551-0_14
Manne S, Kondru N, Jin H, Serrano GE, Anantharam V, Kanthasamy A, Adler CH, Beach TG, et al (2020) Blinded RT-QuIC Analysis of α-Synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov Disord 35(12):2230–2239. https://doi.org/10.1002/mds.28242
Wang Z, Becker K, Donadio V, Siedlak S, Yuan J, Rezaee M, Incensi A, Kuzkina A, et al (2020) Skin α-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol 78(1):30–40. https://doi.org/10.1001/jamaneurol.2020.3311
Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, Johnson LM, Onwubiko HA, Priola SA, et al (2008) Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods 5(3):211–212. https://doi.org/10.1038/nmeth0308-211
Wang N, Gibbons CH, Lafo J, Freeman R (2013) α-Synuclein in cutaneous autonomic nerves. Neurology 81(18):1604–1610. https://doi.org/10.1212/WNL.0b013e3182a9f449
Trivedi DK, Sinclair E, Xu Y, Sarkar D, Walton-Doyle C, Liscio C, Banks P, Milne J, et al (2019) Discovery of volatile biomarkers of Parkinson’s disease from Sebum. ACS Cent Sci 5(4):599–606. https://doi.org/10.1021/acscentsci.8b00879
Kim YJ, Lee CM, Kim S, Jang JW, Lee SY, Lee SH (2019) Risk of Parkinson’s disease after colectomy: longitudinal follow-up study using a national sample cohort. J Neurol. https://doi.org/10.1007/s00415-019-09617-1
Felice VD, Quigley EM, Sullivan AM, O’Keeffe GW, O’Mahony SM (2016) Microbiota-gut-brain signalling in Parkinson’s disease: implications for non-motor symptoms. Parkinsonism Relat Disord 27:1–8. https://doi.org/10.1016/j.parkreldis.2016.03.012
Zhu X, Han Y, Du J, Liu R, Jin K, Yi W (2017) Microbiota-gut-brain axis and the central nervous system. Oncotarget 8 (32):53829–53838. https://doi.org/10.18632/oncotarget.17754
Vlassov AV, Magdaleno S, Setterquist R (1820) Conrad R (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 7:940–948. https://doi.org/10.1016/j.bbagen.2012.03.017
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ (2012) ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40 (Database issue):D1241–1244. https://doi.org/10.1093/nar/gkr828
Proia P, Schiera G, Mineo M, Ingrassia AM, Santoro G, Savettieri G, Di Liegro I (2008) Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int J Mol Med 21(1):63–67
Schiera G, Proia P, Alberti C, Mineo M, Savettieri G, Di Liegro I (2007) Neurons produce FGF2 and VEGF and secrete them at least in part by shedding extracellular vesicles. J Cell Mol Med 11(6):1384–1394. https://doi.org/10.1111/j.1582-4934.2007.00100.x
Pant S, Hilton H, Burczynski ME (2012) The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol 83(11):1484–1494. https://doi.org/10.1016/j.bcp.2011.12.037
Howitt J, Hill AF (2016) Exosomes in the pathology of neurodegenerative diseases. J Biol Chem 291(52):26589–26597. https://doi.org/10.1074/jbc.R116.757955
Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, et al (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128(5):639–650. https://doi.org/10.1007/s00401-014-1314-y
Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener 7:42. https://doi.org/10.1186/1750-1326-7-42
Cao Z, Wu Y, Liu G, Jiang Y, Wang X, Wang Z, Feng T (2019) α-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson’s disease. Neurosci Lett 696:114–120. https://doi.org/10.1016/j.neulet.2018.12.030
Lynge Pedersen AM, Belstrøm D (2019) The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent 80(Suppl 1):S3–S12. https://doi.org/10.1016/j.jdent.2018.08.010
Marsh PD, Do T, Beighton D (2000) Devine DA (2016) Influence of saliva on the oral microbiota. Periodontol 70(1):80–92. https://doi.org/10.1111/prd.12098
Franzosa EA, Morgan XC, Segata N, Waldron L, Reyes J, Earl AM, Giannoukos G, Boylan MR, et al (2014) Relating the metatranscriptome and metagenome of the human gut. Proc Natl Acad Sci U S A 111(22):E2329-2338. https://doi.org/10.1073/pnas.1319284111
Schmidt TS, Hayward MR, Coelho LP, Li SS, Costea PI, Voigt AY, Wirbel J, Maistrenko OM, et al (2019) Extensive transmission of microbes along the gastrointestinal tract. Elife 8. https://doi.org/10.7554/eLife.42693
Mihaila D, Donegan J, Barns S, LaRocca D, Du Q, Zheng D, Vidal M, Neville C, et al (2019) The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS ONE 14(6):e0218252. https://doi.org/10.1371/journal.pone.0218252
Belibasakis GN, Bostanci N, Marsh PD, Zaura E (2019) Applications of the oral microbiome in personalized dentistry. Arch Oral Biol 104:7–12. https://doi.org/10.1016/j.archoralbio.2019.05.023
Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, Coron E, Bruley des Varannes S, et al (2013) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50:42–48. https://doi.org/10.1016/j.nbd.2012.09.007
Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, et al (2009) Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A 106(39):16799–16804. https://doi.org/10.1073/pnas.0906773106
El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A (2002) Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123(5):1607–1615. https://doi.org/10.1053/gast.2002.36578
Wilson CM, McGilligan K, Thomas DW (1988) Determination of fecal alpha 1-antitrypsin concentration by radial immunodiffusion: two systems compared. Clin Chem 34(2):372–376
Silva MT (2010) When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol 87(1):93–106. https://doi.org/10.1189/jlb.0809549
Hessian PA, Edgeworth J, Hogg N (1993) MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol 53(2):197–204
Roth J, Goebeler M, Wrocklage V, van den Bos C, Sorg C (1994) Expression of the calcium-binding proteins MRP8 and MRP14 in monocytes is regulated by a calcium-induced suppressor mechanism. Biochem J 301(Pt 3):655–660. https://doi.org/10.1042/bj3010655
Eue I, König S, Pior J, Sorg C (2002) S100A8, S100A9 and the S100A8/A9 heterodimer complex specifically bind to human endothelial cells: identification and characterization of ligands for the myeloid-related proteins S100A9 and S100A8/A9 on human dermal microvascular endothelial cell line-1 cells. Int Immunol 14(3):287–297. https://doi.org/10.1093/intimm/14.3.287
Clohessy PA, Golden BE (1995) Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol 42(5):551–556. https://doi.org/10.1111/j.1365-3083.1995.tb03695.x
Masson PL, Heremans JF, Schonne E (1969) Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med 130(3):643–658. https://doi.org/10.1084/jem.130.3.643
Summerton CB, Longlands MG, Wiener K, Shreeve DR (2002) Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol 14(8):841–845. https://doi.org/10.1097/00042737-200208000-00005
Guerrant RL, Araujo V, Soares E, Kotloff K, Lima AA, Cooper WH, Lee AG (1992) Measurement of fecal lactoferrin as a marker of fecal leukocytes. J Clin Microbiol 30(5):1238–1242. https://doi.org/10.1128/JCM.30.5.1238-1242.1992
Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Bürmann J, Faßbender K, Schäfer KH, Unger MM (2018) Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord 50:104–107. https://doi.org/10.1016/j.parkreldis.2018.02.022
Mulak A, Koszewicz M, Panek-Jeziorna M, Koziorowska-Gawron E, Budrewicz S (2019) Fecal calprotectin as a marker of the gut immune system activation is elevated in Parkinson’s disease. Front Neurosci 13:992. https://doi.org/10.3389/fnins.2019.00992
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76(3):217–221. https://doi.org/10.1007/BF00687767
Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, Broughton E, Hagan H, et al (2014) Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol 127(2):235–241. https://doi.org/10.1007/s00401-013-1214-6
Yan F, Chen Y, Li M, Wang Y, Zhang W, Chen X, Ye Q (2018) Gastrointestinal nervous system α-synuclein as a potential biomarker of Parkinson disease. Medicine (Baltimore) 97(28):e11337. https://doi.org/10.1097/MD.0000000000011337
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, et al (2011) Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 6(12):e28032. https://doi.org/10.1371/journal.pone.0028032
Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, et al (2011) The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 23(12):1132–1139. https://doi.org/10.1111/j.1365-2982.2011.01796.x
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108(38):16050–16055. https://doi.org/10.1073/pnas.1102999108
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, et al (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167(6):1469-1480.e1412. https://doi.org/10.1016/j.cell.2016.11.018
Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, et al (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18(7):965–977. https://doi.org/10.1038/nn.4030
Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, et al (2011) Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A 108(Suppl 1):4586–4591. https://doi.org/10.1073/pnas.1000097107
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, et al (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72. https://doi.org/10.1016/j.parkreldis.2016.08.019
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28(10):1221–1227. https://doi.org/10.1136/gut.28.10.1221
Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A, Troost FJ, Venema K, et al (2009) Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr 28(1):88–93. https://doi.org/10.1016/j.clnu.2008.11.002
Neunlist M, Dobreva G, Schemann M (1999) Characteristics of mucosally projecting myenteric neurones in the guinea-pig proximal colon. J Physiol 517(Pt 2):533–546. https://doi.org/10.1111/j.1469-7793.1999.0533t.x
Clark A, Mach N (2017) The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol 8:319. https://doi.org/10.3389/fphys.2017.00319
Ma J, Coarfa C, Qin X, Bonnen PE, Milosavljevic A, Versalovic J, Aagaard K (2014) mtDNA haplogroup and single nucleotide polymorphisms structure human microbiome communities. BMC Genomics 15:257. https://doi.org/10.1186/1471-2164-15-257
Giles RE, Blanc H, Cann HM, Wallace DC (1980) Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A 77(11):6715–6719. https://doi.org/10.1073/pnas.77.11.6715
Moeller AH, Suzuki TA, Phifer-Rixey M, Nachman MW (2018) Transmission modes of the mammalian gut microbiota. Science 362(6413):453–457. https://doi.org/10.1126/science.aat7164
Yardeni T, Tanes CE, Bittinger K, Mattei LM, Schaefer PM, Singh LN, Wu GD, Murdock DG, et al (2019) Host mitochondria influence gut microbiome diversity: A role for ROS. Sci Signal 12(588). https://doi.org/10.1126/scisignal.aaw3159
Chahine LM, Stern MB, Chen-Plotkin A (2014) Blood-based biomarkers for Parkinson’s disease. Parkinsonism Relat Disord 20(Suppl 1):S99-103. https://doi.org/10.1016/S1353-8020(13)70025-7
Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, Singleton AB (2004) Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology 62(10):1835–1838. https://doi.org/10.1212/01.wnl.0000127517.33208.f4
Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, et al (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5(2):55–59. https://doi.org/10.1159/000112832
Tian C, Liu G, Gao L, Soltys D, Pan C, Stewart T, Shi M, Xie Z, et al (2019) Erythrocytic α-Synuclein as a potential biomarker for Parkinson’s disease. Transl Neurodegener 8:15. https://doi.org/10.1186/s40035-019-0155-y
Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E (2008) The antioxidant properties of serum albumin. FEBS Lett 582(13):1783–1787. https://doi.org/10.1016/j.febslet.2008.04.057
Ueno SI, Hatano T, Okuzumi A, Saiki S, Oji Y, Mori A, Koinuma T, Fujimaki M, et al (2020) Nonmercaptalbumin as an oxidative stress marker in Parkinson’s and PARK2 disease. Ann Clin Transl Neurol 7(3):307–317. https://doi.org/10.1002/acn3.50990
Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90(8):870–881. https://doi.org/10.1136/jnnp-2018-320106
Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, Zecca C, Blennow K, et al (2017) Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81(6):857–870. https://doi.org/10.1002/ana.24954
Lin YS, Lee WJ, Wang SJ, Fuh JL (2018) Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 8(1):17368. https://doi.org/10.1038/s41598-018-35766-w
Lin CH, Chiu MJ (2020) Author response: Blood NfL: a biomarker for disease severity and progression in Parkinson disease. Neurology 95(14):658. https://doi.org/10.1212/WNL.0000000000010664
Sampedro F, Pérez-González R, Martínez-Horta S, Marín-Lahoz J, Pagonabarraga J, Kulisevsky J (2020) Serum neurofilament light chain levels reflect cortical neurodegeneration in de novo Parkinson’s disease. Parkinsonism Relat Disord 74:43–49. https://doi.org/10.1016/j.parkreldis.2020.04.009
Marques TM, van Rumund A, Oeckl P, Kuiperij HB, Esselink RAJ, Bloem BR, Otto M, Verbeek MM (2019) Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 92(13):e1479–e1486. https://doi.org/10.1212/WNL.0000000000007179
Chung CC, Chan L, Chen JH, Bamodu OA, Hong CT (2020) Neurofilament light chain level in plasma extracellular vesicles and Parkinson’s disease. Ther Adv Neurol Disord 13:1756286420975917. https://doi.org/10.1177/1756286420975917
Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, et al (2017) Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 88(10):930–937. https://doi.org/10.1212/WNL.0000000000003680
Bäckström D, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, Forsgren L, Lenfeldt N (2020) NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology 95(7):e827–e838. https://doi.org/10.1212/WNL.0000000000010084
Mizobuchi N, Hoseki J, Kubota H, Toyokuni S, Nozaki J, Naitoh M, Koizumi A, Nagata K (2007) ARMET is a soluble ER protein induced by the unfolded protein response via ERSE-II element. Cell Struct Funct 32(1):41–50. https://doi.org/10.1247/csf.07001
Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, et al (2003) MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci 20(2):173–188. https://doi.org/10.1385/jmn:20:2:173
Zhang GL, Wang LH, Liu XY, Zhang YX, Hu MY, Liu L, Fang YY, Mu Y, et al (2018) Cerebral dopamine neurotrophic factor (CDNF) has neuroprotective effects against cerebral ischemia that may occur through the endoplasmic reticulum stress pathway. Int J Mol Sci 19(7):1905. https://doi.org/10.3390/ijms19071905
Volmer R, van der Ploeg K, Ron D (2013) Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A 110(12):4628–4633. https://doi.org/10.1073/pnas.1217611110
Sousa-Victor P, Neves J, Cedron-Craft W, Ventura PB, Liao CY, Riley RR, Soifer I, van Bruggen N, et al (2019) MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat Metab 1(2):276–290. https://doi.org/10.1038/s42255-018-0023-6
Galli E, Planken A, Kadastik-Eerme L, Saarma M, Taba P, Lindholm P (2019) Increased serum levels of mesencephalic astrocyte-derived neurotrophic factor in subjects with Parkinson’s disease. Front Neurosci 13:929. https://doi.org/10.3389/fnins.2019.00929
Shang P, Zhang Y, Ma D, Hao Y, Wang X, Xin M, Zhu M, Feng J (2019) Inflammation resolution and specialized pro-resolving lipid mediators in CNS diseases. Expert Opin Ther Targets 23(11):967–986. https://doi.org/10.1080/14728222.2019.1691525
Abdolmaleki F, Kovanen PT, Mardani R, Gheibi-Hayat SM, Bo S, Sahebkar A (2020) Resolvins: emerging players in autoimmune and inflammatory diseases. Clin Rev Allergy Immunol 58(1):82–91. https://doi.org/10.1007/s12016-019-08754-9
Ren YZ, Zhang BZ, Zhao XJ, Zhang ZY (2020) Resolvin D1 ameliorates cognitive impairment following traumatic brain injury via protecting astrocytic mitochondria. J Neurochem 154(5):530–546. https://doi.org/10.1111/jnc.14962
Krashia P, Cordella A, Nobili A, La Barbera L, Federici M, Leuti A, Campanelli F, Natale G, et al (2019) Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat Commun 10(1):3945. https://doi.org/10.1038/s41467-019-11928-w
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47(6 Suppl 3):S161-170. https://doi.org/10.1212/wnl.47.6_suppl_3.161s
Cipriani S, Chen X, Schwarzschild MA (2010) Urate: a novel biomarker of Parkinson’s disease risk, diagnosis and prognosis. Biomark Med 4(5):701–712. https://doi.org/10.2217/bmm.10.94
Hediger MA, Johnson RJ, Miyazaki H, Endou H (2005) Molecular physiology of urate transport. Physiology (Bethesda) 20:125–133. https://doi.org/10.1152/physiol.00039.2004
Yeum KJ, Russell RM, Krinsky NI, Aldini G (2004) Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys 430(1):97–103. https://doi.org/10.1016/j.abb.2004.03.006
de Lau LM, Koudstaal PJ, Hofman A, Breteler MM (2005) Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 58(5):797–800. https://doi.org/10.1002/ana.20663
Church WH, Ward VL (1994) Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain Res Bull 33(4):419–425. https://doi.org/10.1016/0361-9230(94)90285-2
Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM (1996) Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am J Epidemiol 144(5):480–484. https://doi.org/10.1093/oxfordjournals.aje.a008954
Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A (2007) Plasma urate and risk of Parkinson’s disease. Am J Epidemiol 166(5):561–567. https://doi.org/10.1093/aje/kwm127
O’Reilly EJ, Gao X, Weisskopf MG, Chen H, Schwarzschild MA, Spiegelman D, Ascherio A (2010) Plasma urate and Parkinson’s disease in women. Am J Epidemiol 172(6):666–670. https://doi.org/10.1093/aje/kwq195
Gao X, O’Reilly É, Schwarzschild MA, Ascherio A (2016) Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology 86(6):520–526. https://doi.org/10.1212/WNL.0000000000002351
Cantoni GL (1953) The nature of the active methyl donor formed enzymatically from L-methionine and adenosinetriphosphate. J Amer Chem Soc 74:2942–2943. https://doi.org/10.1021/ja01131a519
Silla Y, Varshney S, Ray A, Basak T, Zinellu A, Sabareesh V, Carru C, Sengupta S (2019) Hydrolysis of homocysteine thiolactone results in the formation of Protein-Cys-S-S-homocysteinylation. Proteins 87(8):625–634. https://doi.org/10.1002/prot.25681
Hasan T, Arora R, Bansal AK, Bhattacharya R, Sharma GS, Singh LR (2019) Disturbed homocysteine metabolism is associated with cancer. Exp Mol Med 51(2):1–13. https://doi.org/10.1038/s12276-019-0216-4
Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, Lucca U (2004) Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr 80(1):114–122. https://doi.org/10.1093/ajcn/80.1.114
Kuhn W, Roebroek R, Blom H, van Oppenraaij D, Przuntek H, Kretschmer A, Büttner T, Woitalla D, et al (1998) Elevated plasma levels of homocysteine in Parkinson’s disease. Eur Neurol 40(4):225–227. https://doi.org/10.1159/000007984
Sleeman I, Lawson RA, Yarnall AJ, Duncan GW, Johnston F, Khoo TK, Burn DJ (2019) Urate and homocysteine: predicting motor and cognitive changes in newly diagnosed Parkinson’s disease. J Parkinsons Dis 9(2):351–359. https://doi.org/10.3233/JPD-181535
McLeish MJ, Kenyon GL (2005) Relating structure to mechanism in creatine kinase. Crit Rev Biochem Mol Biol 40(1):1–20. https://doi.org/10.1080/10409230590918577
Eppenberger HM, Dawson DM, Kaplan NO (1967) The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem 242(2):204–209
Wyss M, Smeitink J, Wevers RA, Wallimann T (1992) Mitochondrial creatine kinase: a key enzyme of aerobic energy metabolism. Biochim Biophys Acta 1102(2):119–166. https://doi.org/10.1016/0005-2728(92)90096-k
Schlattner U, Tokarska-Schlattner M, Wallimann T (2006) Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta 1762(2):164–180. https://doi.org/10.1016/j.bbadis.2005.09.004
Beal MF (2000) Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci 23(7):298–304. https://doi.org/10.1016/s0166-2236(00)01584-8
Xu J, Fu X, Pan M, Zhou X, Chen Z, Wang D, Zhang X, Chen Q, et al (2019) Mitochondrial creatine kinase is decreased in the serum of idiopathic Parkinson’s disease patients. Aging Dis 10(3):601–610. https://doi.org/10.14336/AD.2018.0615
Bujak R, Struck-Lewicka W, Markuszewski MJ, Kaliszan R (2015) Metabolomics for laboratory diagnostics. J Pharm Biomed Anal 113:108–120. https://doi.org/10.1016/j.jpba.2014.12.017
Bogdanov M, Matson WR, Wang L, Matson T, Saunders-Pullman R, Bressman SS, Flint Beal M (2008) Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain 131(Pt 2):389–396. https://doi.org/10.1093/brain/awm304
Ahmed SS, Santosh W, Kumar S, Christlet HT (2009) Metabolic profiling of Parkinson’s disease: evidence of biomarker from gene expression analysis and rapid neural network detection. J Biomed Sci 16:63. https://doi.org/10.1186/1423-0127-16-63
Trupp M, Jonsson P, Ohrfelt A, Zetterberg H, Obudulu O, Malm L, Wuolikainen A, Linder J, et al (2014) Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. J Parkinsons Dis 4(3):549–560. https://doi.org/10.3233/JPD-140389
Yakhine-Diop SMS, Morales-García JA, Niso-Santano M, González-Polo RA, Uribe-Carretero E, Martinez-Chacon G, Durand S, Maiuri MC, et al (2020) Metabolic alterations in plasma from patients with familial and idiopathic Parkinson’s disease. Aging (Albany NY) 12(17):16690–16708. https://doi.org/10.18632/aging.103992
Shao Y, Li T, Liu Z, Wang X, Xu X, Li S, Xu G, Le W (2021) Comprehensive metabolic profiling of Parkinson’s disease by liquid chromatography-mass spectrometry. Mol Neurodegener 16(1):4. https://doi.org/10.1186/s13024-021-00425-8
Johansen KK, Wang L, Aasly JO, White LR, Matson WR, Henchcliffe C, Beal MF, Bogdanov M (2009) Metabolomic profiling in LRRK2-related Parkinson’s disease. PLoS ONE 4(10):e7551. https://doi.org/10.1371/journal.pone.0007551
Grosshans H, Slack FJ (2002) Micro-RNAs: small is plentiful. J Cell Biol 156(1):17–21. https://doi.org/10.1083/jcb.200111033
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75(5):843–854. https://doi.org/10.1016/0092-8674(93)90529-y
Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, Lassmann H, Wekerle H, et al (2009) MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 132(Pt 12):3342–3352. https://doi.org/10.1093/brain/awp300
Femminella GD, Ferrara N, Rengo G (2015) The emerging role of microRNAs in Alzheimer’s disease. Front Physiol 6:40. https://doi.org/10.3389/fphys.2015.00040
Behbahanipour M, Peymani M, Salari M, Hashemi MS, Nasr-Esfahani MH, Ghaedi K (2019) Expression profiling of blood microRNAs 885, 361, and 17 in the patients with the Parkinson’s disease: integrating interaction data to uncover the possible triggering age-related mechanisms. Sci Rep 9(1):13759. https://doi.org/10.1038/s41598-019-50256-3
Wu J, Gao Y (2015) Physiological conditions can be reflected in human urine proteome and metabolome. Expert Rev Proteomics 12(6):623–636. https://doi.org/10.1586/14789450.2015.1094380
Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24(8). https://doi.org/10.3390/molecules24081583
Sato S, Mizuno Y, Hattori N (2005) Urinary 8-hydroxydeoxyguanosine levels as a biomarker for progression of Parkinson disease. Neurology 64(6):1081–1083. https://doi.org/10.1212/01.WNL.0000154597.24838.6B
Hirayama M, Nakamura T, Watanabe H, Uchida K, Hama T, Hara T, Niimi Y, Ito M, et al (2011) Urinary 8-hydroxydeoxyguanosine correlate with hallucinations rather than motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 17(1):46–49. https://doi.org/10.1016/j.parkreldis.2010.11.004
Tan L, Yu JT (2012) The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. J Neurol Sci 323(1–2):1–8. https://doi.org/10.1016/j.jns.2012.08.005
Bai JH, Zheng YL, Yu YP (2020) Urinary kynurenine as a biomarker for Parkinson’s disease. Neurol Sci. https://doi.org/10.1007/s10072-020-04589-x
Luan H, Liu LF, Meng N, Tang Z, Chua KK, Chen LL, Song JX, Mok VC, et al (2015) LC-MS-based urinary metabolite signatures in idiopathic Parkinson’s disease. J Proteome Res 14(1):467–478. https://doi.org/10.1021/pr500807t
Wang S, Kojima K, Mobley JA, West AB (2019) Proteomic analysis of urinary extracellular vesicles reveal biomarkers for neurologic disease. EBioMedicine 45:351–361. https://doi.org/10.1016/j.ebiom.2019.06.021
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, et al (2017) Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32(5):739–749. https://doi.org/10.1002/mds.26942
Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Tanner C, Marek K, Investigators P (2016) How stable are Parkinson’s disease subtypes in de novo patients: Analysis of the PPMI cohort? Parkinsonism Relat Disord 28:62–67. https://doi.org/10.1016/j.parkreldis.2016.04.027
Acknowledgements
Y.G.C. (No. CVU: 856246) and A.P.D.J. (No. CVU: 708195) received a scholarship from the National Council of Science and Technology (Consejo Nacional de Ciencia y Tecnología, CONACYT). All figures were created with Biorender.com.
Funding
This research has been funded by Programa de Apoyo a la Investigación Científica y Tecnológica (PAICyT) 2021: SA1872-21 (A.G.G), and SA1891-21 (H.R.R).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gopar-Cuevas, Y., Duarte-Jurado, A.P., Diaz-Perez, R.N. et al. Pursuing Multiple Biomarkers for Early Idiopathic Parkinson’s Disease Diagnosis. Mol Neurobiol 58, 5517–5532 (2021). https://doi.org/10.1007/s12035-021-02500-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02500-z