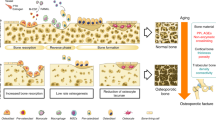
Abstract
The human skeleton has remarkable regenerative properties, being one of the few structures in the body that can heal by recreating its normal cellular composition, orientation and mechanical strength. When the healing process of a fractured bone fails owing to inadequate immobilization, failed surgical intervention, insufficient biological response or infection, the outcome after a prolonged period of no healing is defined as non-union. Non-union represents a chronic medical condition not only affecting function but also potentially impacting the individual’s psychosocial and economic well-being. This Primer provides the reader with an in-depth understanding of our contemporary knowledge regarding the important features to be considered when faced with non-union. The normal mechanisms involved in bone healing and the factors that disrupt the normal signalling mechanisms are addressed. Epidemiological considerations and advances in the diagnosis and surgical therapy of non-union are highlighted and the need for greater efforts in basic, translational and clinical research are identified.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Brinker, M. R., Hanus, B. D., Sen, M. & O’Connor, D. P. The devastating effects of tibial nonunion on health-related quality of life. J. Bone Joint Surg. Am. 95, 2170–2176 (2013).
Brinker, M. R., Trivedi, A. & O’Connor, D. P. Debilitating effects of femoral nonunion on health-related quality of life. J. Orthop. Trauma 31, e37–e42 (2017). A cohort study looking at femoral non-union, the impact of this disease is similar to end-stage hip arthrosis and tibial non-union, and worse than other medical conditions.
Mills, L. A., Aitken, S. A. & Simpson, A. The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults. Acta Orthop. 88, 434–439 (2017). This paper showed that the analysis of non-union incidence revealed the overall peak incidence at an age between 30 and 40 years, with more frequent non-unions in upper limbs than in lower limbs.
Zura, R. et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 151, e162775 (2016). This paper is a large cohort study identifying patient-specific risk factors for the development of non-union.
Taylor, J. C. in Campbell’s Operative Orthopaedics (eds Crenshaw, A. H., Daugherty, K. & Campbell, W. C.) 1287–1345 (Mosby, 1992).
Brinker, M. R. & O’Connor, D. P. in Skeletal Trauma (eds Browner, B., Jupiter, J., Krettek, C. & Anderson, P.) 743–834 (Elsevier, 2020).
Weber, B. G. & Cech, O. Pseudarthrosis: Pathophysiology, Biomechanics, Therapy, Results (Grune & Stratton, 1976). This book describes the classification of non-union types, which is still used today.
Schottel, P. C., O’Connor, D. P. & Brinker, M. R. Time trade-off as a measure of health-related quality of life: long bone nonunions have a devastating impact. J. Bone Joint Surg. Am. 97, 1406–1410 (2015).
Hak, D. J. et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury 45 (Suppl. 2), S3–S7 (2014).
Ekegren, C. L., Edwards, E. R., de Steiger, R. & Gabbe, B. J. Incidence, costs and predictors of non-union, delayed union and mal-union following long bone fracture. Int J. Environ. Res Public Health 15, 2845 (2018). This is an analysis of the Victorian Orthopaedic Trauma Outcomes Registry showing that older age and tibia or femoral shaft fracture are risk factors for the development of non-union.
Özkan, S., Nolte, P. A., van den Bekerom, M. P. J. & Bloemers, F. W. Diagnosis and management of long-bone nonunions: a nationwide survey. Eur. J. Trauma Emerg. Surg. 45, 3–11 (2019).
Mathieu, L. et al. Management of the complications of traditional bone setting for upper extremity fractures: the experiences of a French Forward Surgical Team in Chad. Chirur. Main. 33, 137–143 (2014).
Reynolds, T. A. et al. The impact of trauma care systems in low- and middle-income countries. Annu. Rev. Public. Health 38, 507–532 (2017).
Bhandari, M. et al. A lack of consensus in the assessment of fracture healing among orthopaedic surgeons. J. Orthop. Trauma 16, 562–566 (2002).
Tian, R. et al. Prevalence and influencing factors of nonunion in patients with tibial fracture: systematic review and meta-analysis. J. Orthop. Surg. Res. 15, 377 (2020).
Tzioupis, C. & Giannoudis, P. V. Prevalence of long-bone non-unions. Injury 38 (Suppl. 2), S3–S9 (2007).
Keating, J. F., O’Brien, P. I., Blachut, P., Meek, R. N. & Broekhuyse, H. M. Reamed interlocking intramedullary nailing of open fractures of the tibia. Clin. Orthop. Relat. Res. 338, 182–191 (1997).
O’Halloran, K. et al. Will my tibial fracture heal? predicting nonunion at the time of definitive fixation based on commonly available variables. Clin. Orthop. Relat. Res. 474, 1385–1395 (2016).
Ross, K. A. et al. Prediction of tibial nonunion at the 6-week time point. Injury 49, 2075–2082 (2018).
Canadian Orthopaedic Trauma Society.Nonunion following intramedullary nailing of the femur with and without reaming: results of a multicenter randomized clinical trial. J. Bone Joint Surg. Am. 85, 2093–2096 (2003).
Metsemakers, W. J., Roels, N., Belmans, A., Reynders, P. & Nijs, S. Risk factors for nonunion after intramedullary nailing of femoral shaft fractures: remaining controversies. Injury 46, 1601–1607 (2015).
Rommens, P. M., Blum, J. & Runkel, M. Retrograde nailing of humeral shaft fractures. Clin. Orthop. Relat. Res. 350, 26–39 (1998).
Sarmiento, A., Zagorski, J. B., Zych, G. A., Latta, L. L. & Capps, C. A. Functional bracing for the treatment of fractures of the humeral diaphysis. J. Bone Joint Surg. Am. 82, 478–486 (2000).
Singh, A. et al. Gustilo IIIB open tibial fractures: an analysis of infection and nonunion rates. Indian J. Orthop. 52, 406–410 (2018).
Calori, G. M., Albisetti, W., Agus, A., Iori, S. & Tagliabue, L. Risk factors contributing to fracture non-unions. Injury 38 (Suppl. 2), S11–S18 (2007).
Taitsman, L. A., Lynch, J. R., Agel, J., Barei, D. P. & Nork, S. E. Risk factors for femoral nonunion after femoral shaft fracture. J. Trauma 67, 1389–1392 (2009).
Winquist, R. A., Hansen, J. S. T. & Clawson, D. K. Closed intramedullary nailing of femoral fractures. A report of five hundred and twenty cases. 1984. J. Bone Joint Surg. Am. 83, 1912–1912 (2001).
Ma, Y.-G., Hu, G.-L., Hu, W. & Liang, F. Surgical factors contributing to nonunion in femoral shaft fracture following intramedullary nailing. Chin. J. Traumatol. 19, 109–112 (2016).
Mills, L. A. & Simpson, A. H. R. W. The relative incidence of fracture non-union in the Scottish population (5.17 million): a 5-year epidemiological study. BMJ Open 3, e002276 (2013).
Zura, R. et al. Bone fracture nonunion rate decreases with increasing age: a prospective inception cohort study. Bone 95, 26–32 (2017).
Scolaro, J. A. et al. Cigarette smoking increases complications following fracture. J. Bone Joint Surg. Am. 96, 674–681 (2014).
Hernigou, J. & Schuind, F. Smoking as a predictor of negative outcome in diaphyseal fracture healing. Int. Orthop. 37, 883–887 (2013).
Jeffcoach, D. R. et al. Nonsteroidal anti-inflammatory drugs’ impact on nonunion and infection rates in long-bone fractures. J. Trauma Acute Care Surg. 76, 779–783 (2014).
Moghaddam, A. et al. Cigarette smoking influences the clinical and occupational outcome of patients with tibial shaft fractures. Injury 42, 1435–1442 (2011).
Pearson, R. G., Clement, R. G. E., Edwards, K. L. & Scammell, B. E. Do smokers have greater risk of delayed and non-union after fracture, osteotomy and arthrodesis? A systematic review with meta-analysis. BMJ Open 6, e010303 (2016).
Zura, R., Mehta, S., Della Rocca, G. J. & Steen, R. G. Biological risk factors for nonunion of bone fracture. JBJS Rev. 4, 1 (2016).
Dodwell, E. R. et al. NSAID exposure and risk of nonunion: a meta-analysis of case–control and cohort studies. Calcif. Tissue Int. 87, 193–202 (2010).
Hernandez, R. K., Do, T. P., Critchlow, C. W., Dent, R. E. & Jick, S. S. Patient-related risk factors for fracture-healing complications in the United Kingdom general practice research database. Acta Orthop. 83, 653–660 (2012).
Burrus, M. T., Werner, B. C. & Yarboro, S. R. Obesity is associated with increased postoperative complications after operative management of tibial shaft fractures. Injury 47, 465–470 (2015).
Gortler, H. et al. Diabetes and healing outcomes in lower extremity fractures: a systematic review. Injury 49, 177–183 (2018).
van Wunnik, B. P. W., Weijers, P. H. E., van Helden, S. H., Brink, P. R. G. & Poeze, M. Osteoporosis is not a risk factor for the development of nonunion: a cohort nested case–control study. Injury 42, 1491–1494 (2011).
Brinker, M. R., O’Connor, D. P., Monla, Y. T. & Earthman, T. P. Metabolic and endocrine abnormalities in patients with nonunions. Curr. Orthop. Pract. 19, 430–442 (2008).
Dimitriou, R., Carr, I. M., West, R. M., Markham, A. F. & Giannoudis, P. V. Genetic predisposition to fracture non-union: a case control study of a preliminary single nucleotide polymorphisms analysis of the BMP pathway. BMC Musculoskelet. Disord. 12, 44–44 (2011).
Guimarães, J. M. et al. Polymorphisms in BMP4 and FGFR1 genes are associated with fracture non-union: polymorphisms and non-union. J. Orthop. Res. 31, 1971–1979 (2013).
Sathyendra, V. M. D. et al. Single nucleotide polymorphisms in osteogenic genes in atrophic delayed fracture-healing. J. Bone Joint Surg. Am. 96, 1242–1248 (2014).
Zeckey, C. et al. Are polymorphisms of molecules involved in bone healing correlated to aseptic femoral and tibial shaft non-unions? J. Orthop. Res. 29, 1724–1731 (2011).
McCoy, J. T. H. et al. Genomewide association study of fracture nonunion using electronic health records. JBMR 3, 23–28 (2019).
Aslan, A. et al. Surgical treatment of osteopetrosis-related femoral fractures: two case reports and literature review. Case Rep. Orthop. 2014, 891963 (2014).
Rovira Martí, P. & Ullot Font, R. Orthopaedic disorders of pycnodysostosis: a report of five clinical cases. Int. Orthop. 40, 2221–2231 (2016).
Azzam, K. A., Rush, E. T., Burke, B. R., Nabower, A. M. & Esposito, P. W. Mid-term results of femoral and tibial osteotomies and Fassier-Duval nailing in children with osteogenesis imperfecta. J. Pediatr. Orthop. 38, 331–336 (2018).
Mills, L., Tsang, J., Hopper, G., Keenan, G. & Simpson, A. H. R. W. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Joint Res. 5, 512–519 (2016).
Hofmann, A. et al. Cell viability, osteoblast differentiation, and gene expression are altered in human osteoblasts from hypertrophic fracture non-unions. Bone 42, 894–906 (2008).
Ding, Z. C., Lin, Y. K., Gan, Y. K. & Tang, T. T. Molecular pathogenesis of fracture nonunion. J. Orthop. Transl. 14, 45–56 (2018).
Hellwinkel, J. E., Miclau, T. 3rd, Provencher, M. T., Bahney, C. S. & Working, Z. M. The life of a fracture: biologic progression, healing gone awry, and evaluation of union. JBJS Rev. 8, e1900221 (2020).
Tsiridis, E., Upadhyay, N. & Giannoudis, P. Molecular aspects of fracture healing: which are the important molecules? Injury 38 (Suppl. 1), S11–S25 (2007).
Zhou, X. et al. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 10, e1004820 (2014).
Berendsen, A. D. & Olsen, B. R. Bone development. Bone 80, 14–18 (2015).
Kronenberg, H. M. Developmental regulation of the growth plate. Nature 423, 332–336 (2003).
Boudin, E., Fijalkowski, I., Hendrickx, G. & Van Hul, W. Genetic control of bone mass. Mol. Cell. Endocrinol. 432, 3–13 (2016).
Bonewald, L. F. The amazing osteocyte. J. Bone Min. Res. 26, 229–238 (2011).
Einhorn, T. A. & Gerstenfeld, L. C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 11, 45–54 (2015). This paper is a review on the cellular and molecular processes during fracture healing and the role of the immune system and vascularization but also discusses the impact of the injury and treatment options.
Claes, L., Recknagel, S. & Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8, 133–143 (2012). This review summarizes the cellular processes during fracture healing, the involvement of the immune system and vascularization, and the importance of biomechanical conditions.
Loi, F. et al. Inflammation, fracture and bone repair. Bone 86, 119–130 (2016). This paper summarizes the factors influencing bone healing and discusses possibilities to enhance bone repair by the modulation of the inflammatory phase.
Perren, S. M. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J. Bone Joint Surg. Br. 84, 1093–1110 (2002).
Choy, M. H. V. et al. How much do we know about the role of osteocytes in different phases of fracture healing? A systematic review. J. Orthop. Transl. 21, 111–121 (2020).
Kolar, P. et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. B Rev. 16, 427–434 (2010).
Kovtun, A. et al. The crucial role of neutrophil granulocytes in bone fracture healing. Eur. Cell Mater. 32, 152–162 (2016).
Sinder, B. P., Pettit, A. R. & McCauley, L. K. Macrophages: their emerging roles in bone. J. Bone Miner. Res. 30, 2140–2149 (2015).
Schlundt, C. et al. Individual effector/regulator T cell ratios impact bone regeneration. Front. Immunol. 10, 1954 (2019).
Schmidt-Bleek, K., Kwee, B. J., Mooney, D. J. & Duda, G. N. Boon and bane of inflammation in bone tissue regeneration and its link with angiogenesis. Tissue Eng. B Rev. 21, 354–364 (2015).
Bahney, C. S. et al. Cellular biology of fracture healing. J. Orthop. Res. 37, 35–50 (2019). This paper gives an overview of the cellular and molecular processes during fracture healing.
Claes, L. E. et al. Effects of mechanical factors on the fracture healing process. Clin. Orthop. Relat. Res. 355 (Suppl.), S132–S147 (1998).
Claes, L. E. & Heigele, C. A. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 32, 255–266 (1999).
Perren, S. M. & Boitzy, A. Cellular differentiation and bone biomechanics during the consolidation of a fracture. Anat. Clin. 28, 13–28 (1978). This is an animal study investigating the effects of force and motion on bone healing, which provides important insights into the effects of compression and interfragmentary movement on the healing process.
Perren, S. M. & Rahn, B. A. Biomechanics of fracture healing. Can. J. Surg. 23, 228–232 (1980).
Elliott, D. S. et al. A unified theory of bone healing and nonunion: BHN theory. Bone Joint J. 98-B, 884–891 (2016). This article presents a theory combining knowledge from biological and mechanical aspects of bone formation and healing with the bone-healing unit, which is disturbed in non-unions due to mechanical conditions.
Colnot, C., Zhang, X. & Knothe Tate, M. L. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J. Orthop. Res. 30, 1869–1878 (2012).
Bassett, C. A. & Herrmann, I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature 190, 460–461 (1961).
Grimes, R., Jepsen, K. J., Fitch, J. L., Einhorn, T. A. & Gerstenfeld, L. C. The transcriptome of fracture healing defines mechanisms of coordination of skeletal and vascular development during endochondral bone formation. J. Bone Min. Res. 26, 2597–2609 (2011).
Aghajanian, P. & Mohan, S. The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 6, 19 (2018).
Claes, L. et al. Metaphyseal fracture healing follows similar biomechanical rules as diaphyseal healing. J. Orthop. Res. 29, 425–432 (2011).
Sandberg, O. H. & Aspenberg, P. Inter-trabecular bone formation: a specific mechanism for healing of cancellous bone. Acta Orthop. 87, 459–465 (2016).
Giannoudis, P. V., Einhorn, T. A. & Marsh, D. Fracture healing: the diamond concept. Injury 38 (Suppl. 4), S3–S6 (2007). This paper highlights the importance of the mechanical environment, growth factors, scaffolds and osteogenic cells for successful bone healing and is used for planning the management of fractures.
Andrzejowski, P. & Giannoudis, P. V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 20, 21 (2019).
Claes, L. Improvement of clinical fracture healing — what can be learned from mechano-biological research? J. Biomech. 115, 110148 (2021).
Augat, P. et al. Shear movement at the fracture site delays healing in a diaphyseal fracture model. J. Orthop. Res. 21, 1011–1017 (2003).
Claes, L. E. & Meyers, N. The direction of tissue strain affects the neovascularization in the fracture-healing zone. Med. Hypotheses 137, 109537 (2020).
Bhandari, M. et al. Predictors of reoperation following operative management of fractures of the tibial shaft. J. Orthop. Trauma 17, 353–361 (2003).
Perren, S. M. Physical and biological aspects of fracture healing with special reference to internal fixation.Clin. Orthop. Relat. Res. 138, 175–196 (1979).
Stewart, S., Darwood, A., Masouros, S., Higgins, C. & Ramasamy, A. Mechanotransduction in osteogenesis. Bone Joint Res. 9, 1–14 (2020).
Huang, C. & Ogawa, R. Mechanotransduction in bone repair and regeneration. FASEB J. 24, 3625–3632 (2010).
Wehrle, E. et al. The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis. Model. Mech. 8, 93–104 (2015).
Borgiani, E. et al. Age-related changes in the mechanical regulation of bone healing are explained by altered cellular mechanoresponse. J. Bone Miner. Res. 34, 1923–1937 (2019).
Maruyama, M. et al. Modulation of the inflammatory response and bone healing. Front. Endocrinol. 11, 386 (2020).
Clark, D., Nakamura, M., Miclau, T. & Marcucio, R. Effects of aging on fracture healing. Curr. Osteoporos. Rep. 15, 601–608 (2017).
George, M. D. et al. Risk of nonunion with nonselective NSAIDs, COX-2 inhibitors, and opioids. J. Bone Joint Surg. Am. 102, 1230–1238 (2020).
Miclau, K. R. et al. Stimulating fracture healing in ischemic environments: does oxygen direct stem cell fate during fracture healing? Front. Cell Dev. Biol. 5, 45 (2017).
Santolini, E. et al. Femoral and tibial blood supply: a trigger for non-union? Injury 45, 1665–1673 (2014).
Hankenson, K. D., Dishowitz, M., Gray, C. & Schenker, M. Angiogenesis in bone regeneration. Injury 42, 556–561 (2011).
Weiss, S., Zimmermann, G., Pufe, T., Varoga, D. & Henle, P. The systemic angiogenic response during bone healing. Arch. Orthop. Trauma Surg. 129, 989–997 (2009).
Garcia, P. et al. Rodent animal models of delayed bone healing and non-union formation: a comprehensive review. Eur. Cell Mater. 26, 1–12 (2013).
Reed, A. A., Joyner, C. J., Brownlow, H. C. & Simpson, A. H. Human atrophic fracture non-unions are not avascular. J. Orthop. Res. 20, 593–599 (2002).
Schwabe, P., Simon, P., Kronbach, Z., Schmidmaier, G. & Wildemann, B. A pilot study investigating the histology and growth factor content of human non-union tissue. Int. Orthop. 38, 2623–2629 (2014).
Duchamp de Lageneste, O. et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by periostin. Nat. Commun. 9, 773 (2018).
Abou-Khalil, R. et al. Role of muscle stem cells during skeletal regeneration. Stem Cell 33, 1501–1511 (2015).
Bajada, S., Marshall, M. J., Wright, K. T., Richardson, J. B. & Johnson, W. E. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone 45, 726–735 (2009).
El-Jawhari, J. J. et al. Defective proliferation and osteogenic potential with altered immunoregulatory phenotype of native bone marrow-multipotential stromal cells in atrophic fracture non-union. Sci. Rep. 9, 17340 (2019).
Wagner, J. M. et al. Inflammatory processes and elevated osteoclast activity chaperon atrophic non-union establishment in a murine model. J. Transl. Med. 17, 416 (2019).
Solomon, D. H., Hochberg, M. C., Mogun, H. & Schneeweiss, S. The relation between bisphosphonate use and non-union of fractures of the humerus in older adults. Osteoporos. Int. 20, 895–901 (2009).
Metsemakers, W. J. et al. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 49, 511–522 (2018).
Szczęsny, G. et al. Genetic factors responsible for long bone fractures non-union. Arch. Orthop. Trauma Surg. 131, 275–281 (2011).
Dapunt, U. et al. Are atrophic long-bone nonunions associated with low-grade infections? Ther. Clin. Risk Manag. 11, 1843–1852 (2015).
Seebach, E. & Kubatzky, K. F. Chronic implant-related bone infections — can immune modulation be a therapeutic strategy? Front. Immunol. 10, 1724 (2019).
Fisher, J. S., Kazam, J. J., Fufa, D. & Bartolotta, R. J. Radiologic evaluation of fracture healing. Skelet. Radiol. 48, 349–361 (2019).
Morshed, S., Corrales, L., Genant, H. & Miclau, T. 3rd Outcome assessment in clinical trials of fracture-healing. J. Bone Joint Surg. Am. 90 (Suppl. 1), 62–67 (2008).
Whelan, D. B. et al. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J. Trauma 68, 629–632 (2010). Development and reliability testing of non-union radiographic score to assess fracture healing.
Litrenta, J. et al. Determination of radiographic healing: an assessment of consistency using RUST and modified RUST in metadiaphyseal fractures. J. Orthop. Trauma 29, 516–520 (2015).
Oliver, W. M. et al. The Radiographic Union Score for HUmeral fractures (RUSHU) predicts humeral shaft nonunion. Bone Joint J. 101-B, 1300–1306 (2019).
Henley, M. B. & Miller, A. Internally rotated oblique x-ray to assess healing of distal tibial shaft fractures. J. Orthop. Trauma https://doi.org/10.1097/BOT.0b013e3182645c1a (2012).
Bhattacharyya, T. et al. The accuracy of computed tomography for the diagnosis of tibial nonunion. J. Bone Joint Surg. Am. 88, 692–697 (2006).
Oe, K. et al. Quantitative bone single-photon emission computed tomography imaging for uninfected nonunion: comparison of hypertrophic nonunion and non-hypertrophic nonunion. J. Orthop. Surg. Res. 16, 125 (2021).
Gholamrezanezhad, A. et al. Clinical nononcologic applications of PET/CT and PET/MRI in musculoskeletal, orthopedic, and rheumatologic imaging. AJR Am. J. Roentgenol. 210, W245–W263 (2018).
Moed, B. R. et al. Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: clinical results. J. Orthop. Trauma 12, 206–213 (1998).
Cadet, E. R., Yin, B., Schulz, B., Ahmad, C. S. & Rosenwasser, M. P. Proximal humerus and humeral shaft nonunions. J. Am. Acad. Orthop. Surg. 21, 538–547 (2013).
Nauth, A. et al. Principles of nonunion management: state of the art. J. Orthop. Trauma 32 (Suppl. 1), S52–S57 (2018). This paper gives an overview of the risk factors for the development of non-union, diagnosis of the cause and treatment options.
Bell, A., Templeman, D. & Weinlein, J. C. Nonunion of the femur and tibia: an update. Orthop. Clin. North Am. 47, 365–375 (2016).
Calori, G. M., Phillips, M., Jeetle, S., Tagliabue, L. & Giannoudis, P. V. Classification of non-union: need for a new scoring system? Injury 39 (Suppl. 2), S59–S63 (2008). This paper describes the development of a scoring system that is intended to facilitate decisions for the further treatment of non-unions.
Rammelt, S. & Zwipp, H. Corrective arthrodeses and osteotomies for post-traumatic hindfoot malalignment: indications, techniques, results. Int. Orthop. 37, 1707–1717 (2013).
Peschiera, V., Staletti, L., Cavanna, M., Saporito, M. & Berlusconi, M. Predicting the failure in distal femur fractures. Injury 49 (Suppl. 3), S2–S7 (2018).
Driesman, A. S., Fisher, N., Karia, R., Konda, S. & Egol, K. A. Fracture site mobility at 6 weeks after humeral shaft fracture predicts nonunion without surgery. J. Orthop. Trauma 31, 657–662 (2017).
Nicholson, J. A. et al. Displaced midshaft clavicle fracture union can be accurately predicted with a delayed assessment at 6 weeks following injury: a prospective cohort study. J. Bone Joint Surg. Am. 102, 557–566 (2020).
Nicholson, J. A., Oliver, W. M., MacGillivray, T. J., Robinson, C. M. & Simpson, A. Sonographic bridging callus at six weeks following displaced midshaft clavicle fracture can accurately predict healing. Bone Joint Res. 10, 113–121 (2021).
Brinker, M. R. & O’Connor, D. P. Management of aseptic tibial and femoral diaphyseal nonunions without bony defects. Orthop. Clin. North Am. 47, 67–75 (2016).
Rupp, M. et al. Diaphyseal long bone nonunions - types, aetiology, economics, and treatment recommendations. Int. Orthop. 42, 247–258 (2018). This paper provides an overview of the epidemiology, costs, aetiology, classification and treatment options of non-unions.
Reed, A. A., Joyner, C. J., Isefuku, S., Brownlow, H. C. & Simpson, A. H. Vascularity in a new model of atrophic nonunion. J. Bone Joint Surg. Br. 85, 604–610 (2003).
Panteli, M., Pountos, I., Jones, E. & Giannoudis, P. V. Biological and molecular profile of fracture non-union tissue: current insights. J. Cell. Mol. Med. 19, 685–713 (2015).
Ramoutar, D. N., Rodrigues, J., Quah, C., Boulton, C. & Moran, C. G. Judet decortication and compression plate fixation of long bone non-union: Is bone graft necessary? Injury 42, 1430–1434 (2011).
Amorosa, L. F. et al. A single-stage treatment protocol for presumptive aseptic diaphyseal nonunions: a review of outcomes. J. Orthop. Trauma 27, 582–586 (2013).
Kothari, A., Monk, P. & Handley, R. Percutaneous strain reduction screws-a safe and simple surgical option for problems with bony union. a technical trick. J. Orthop. Trauma 33, e151–e157 (2019).
Wolff, J. Das Gesetz der Transformation der Knochen (Hirschwald, 1892).
Frost, H. M. Bone “mass” and the “mechanostat”: a proposal. Anat. Rec. 219, 1–9 (1987).
Cheal, E. J., Mansmann, K. A., DiGioia, A. M. 3rd, Hayes, W. C. & Perren, S. M. Role of interfragmentary strain in fracture healing: ovine model of a healing osteotomy. J. Orthop. Res. 9, 131–142 (1991).
Palomares, K. T. et al. Mechanical stimulation alters tissue differentiation and molecular expression during bone healing. J. Orthop. Res. 27, 1123–1132 (2009).
Judet, P. R. & Patel, A. Muscle pedicle bone grafting of long bones by osteoperiosteal decortication. Clin. Orthop. Relat. Res. 87, 74–80 (1972).
Somford, M. P., van den Bekerom, M. P. & Kloen, P. Operative treatment for femoral shaft nonunions, a systematic review of the literature. Strateg. Trauma Limb Reconstr. 8, 77–88 (2013).
Bhan, K., Tyagi, A., Kainth, T., Gupta, A. & Umar, M. Reamed exchange nailing in nonunion of tibial shaft fractures: a review of the current evidence. Cureus 12, e9267 (2020).
Hierholzer, C. & Bühren, V. in Intramedullary Nailing, a comprehensive Guide Vol. 1 (eds Rommens, P. M. & Hessmann, M. H.) Ch. 25, 419–452 (Springer, 2015).
Glatt, V., Evans, C. H. & Stoddart, M. J. Regenerative rehabilitation: the role of mechanotransduction in orthopaedic regenerative medicine. J. Orthop. Res. 37, 1263–1269 (2019).
Ilizarov, G. A. The principles of the Ilizarov method. Bull. Hosp. Joint Dis. Orthop. Inst. 48, 1–11 (1988).
Aktuglu, K., Erol, K. & Vahabi, A. Ilizarov bone transport and treatment of critical-sized tibial bone defects: a narrative review. J. Orthop. Traumatol. 20, 22 (2019).
Glatt, V., Tepic, S. & Evans, C. Reverse dynamization: a novel approach to bone healing. J. Am. Acad. Orthop. Surg. 24, e60–e61 (2016).
Glatt, V., Samchukov, M., Cherkashin, A. & Iobst, C. Reverse dynamization accelerates bone-healing in a large-animal osteotomy model. J. Bone Joint Surg. Am. 103, 257–263 (2021).
Gebauer, D., Mayr, E., Orthner, E. & Ryaby, J. P. Low-intensity pulsed ultrasound: effects on nonunions. Ultrasound Med. Biol. 31, 1391–1402 (2005).
Rutten, S., Nolte, P. A., Guit, G. L., Bouman, D. E. & Albers, G. H. Use of low-intensity pulsed ultrasound for posttraumatic nonunions of the tibia: a review of patients treated in the Netherlands. J. Trauma 62, 902–908 (2007).
Elster, E. A. et al. Extracorporeal shock wave therapy for nonunion of the tibia. J. Orthop. Trauma 24, 133–141 (2010).
Alkhawashki, H. M. Shock wave therapy of fracture nonunion. Injury 46, 2248–2252 (2015).
Maazouz, Y. et al. In vitro measurement of the chemical changes occurring within beta-tricalcium phosphate bone graft substitutes. Acta Biomater. 102, 440–457 (2020).
Zhang, J. et al. Cells responding to surface structure of calcium phosphate ceramics for bone regeneration. J. Tissue Eng. Regen. Med. 11, 3273–3283 (2017).
Zhang, Y., Cheng, X., Jansen, J. A., Yang, F. & van den Beucken, J. Titanium surfaces characteristics modulate macrophage polarization. Mater. Sci. Eng. C. Mater. Biol. Appl. 95, 143–151 (2019).
Schmal, H. et al. Nonunion - consensus from the 4th annual meeting of the Danish Orthopaedic Trauma Society. EFORT Open Rev. 5, 46–57 (2020).
Cox, G., Jones, E., McGonagle, D. & Giannoudis, P. V. Reamer-irrigator-aspirator indications and clinical results: a systematic review. Int. Orthop. 35, 951–956 (2011).
Carragee, E. J., Hurwitz, E. L. & Weiner, B. K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 11, 471–491 (2011).
James, A. W. et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 22, 284–297 (2016).
Liu, X. et al. Delivery of growth factors using a smart porous nanocomposite scaffold to repair a mandibular bone defect. Biomacromolecules 15, 1019–1030 (2014).
Quinlan, E. et al. Development of collagen-hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J. Control. Rel. 198, 71–79 (2015).
Walsh, D. P. et al. Rapid healing of a critical-sized bone defect using a collagen-hydroxyapatite scaffold to facilitate low dose, combinatorial growth factor delivery. J. Tissue Eng. Regen. Med. 13, 1843–1853 (2019).
Glatt, V. et al. Improved healing of large segmental defects in the rat femur by reverse dynamization in the presence of bone morphogenetic protein-2. J. Bone Joint Surg. Am. 94, 2063–2073 (2012).
Canintika, A. F. & Dilogo, I. H. Teriparatide for treating delayed union and nonunion: a systematic review. J. Clin. Orthop. Trauma 11, S107–S112 (2020).
Sato, M. et al. Expression of bone matrix proteins mRNA during distraction osteogenesis. J. Bone Min. Res. 13, 1221–1231 (1998).
Sato, M. et al. Mechanical tension-stress induces expression of bone morphogenetic protein (BMP)-2 and BMP-4, but not BMP-6, BMP-7, and GDF-5 mRNA, during distraction osteogenesis. J. Bone Min. Res. 14, 1084–1095 (1999).
Rauch, F. et al. Temporal and spatial expression of bone morphogenetic protein-2, -4, and -7 during distraction osteogenesis in rabbits. Bone 27, 453–459 (2000).
Radomisli, T. E., Moore, D. C., Barrach, H. J., Keeping, H. S. & Ehrlich, M. G. Weight-bearing alters the expression of collagen types I and II, BMP 2/4 and osteocalcin in the early stages of distraction osteogenesis. J. Orthop. Res. 19, 1049–1056 (2001).
Aspenberg, P., Basic, N., Tagil, M. & Vukicevic, S. Reduced expression of BMP-3 due to mechanical loading: a link between mechanical stimuli and tissue differentiation. Acta Orthop. Scand. 71, 558–562 (2000).
Daluiski, A. et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat. Genet. 27, 84–88 (2001).
Wozney, J. M. & Rosen, V. in Physiology and Pharmacology of Bone 725–748 (Springer, 1993).
Hernigou, P., Poignard, A., Beaujean, F. & Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Joint Surg. Am. 87, 1430–1437 (2005). This paper demonstrates the clinical potential of naive bone marrow progenitor cells.
Garnavos, C., Mouzopoulos, G. & Morakis, E. Fixed intramedullary nailing and percutaneous autologous concentrated bone-marrow grafting can promote bone healing in humeral-shaft fractures with delayed union. Injury 41, 563–567 (2010).
Gomez-Barrena, E. et al. Feasibility and safety of treating non-unions in tibia, femur and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials 196, 100–108 (2019).
Ismail, H. D. et al. Mesenchymal stem cell implantation in atrophic nonunion of the long bones: a translational study. Bone Joint Res. 5, 287–293 (2016).
Liu, S. et al. Manufacturing differences affect human bone marrow stromal cell characteristics and function: comparison of production methods and products from multiple centers. Sci. Rep. 7, 46731 (2017).
Murao, H., Yamamoto, K., Matsuda, S. & Akiyama, H. Periosteal cells are a major source of soft callus in bone fracture. J. Bone Miner. Metab. 31, 390–398 (2013).
Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Min. Res. 24, 274–282 (2009). This paper identified the source and preferred healing pathways of skeletal progenitor cells.
Bragdon, B. C. & Bahney, C. S. Origin of reparative stem cells in fracture healing. Curr. Osteoporos. Rep. 16, 490–503 (2018).
Manzini, B. M., Machado, L. M. R., Noritomi, P. Y. & Da Silva, J. V. L. Advances in bone tissue engineering: a fundamental review. J. Biosci. 46, 17 (2021).
Perez, J. R. et al. Tissue engineering and cell-based therapies for fractures and bone defects. Front. Bioeng. Biotechnol. 6, 105 (2018).
Kon, T. et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Min. Res. 16, 1004–1014 (2001).
Schlundt, C. et al. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone 106, 78–89 (2018).
Reinke, S. et al. Terminally differentiated CD8+ T cells negatively affect bone regeneration in humans. Sci. Transl. Med. 5, 177ra136 (2013). Combination of clinical and preclinical studies identifying a special T cell subtype in impaired bone healing, which might be useful as a possible prognostic marker and therapeutic target.
Julier, Z. et al. Enhancing the regenerative effectiveness of growth factors by local inhibition of interleukin-1 receptor signaling. Sci. Adv. 6, eaba7602 (2020).
Martino, M. M. et al. Inhibition of IL-1R1/MyD88 signalling promotes mesenchymal stem cell-driven tissue regeneration. Nat. Commun. 7, 11051 (2016).
World Health Organization. WHO/WHOQOL: Measuring Quality of Life (2020).
Johnson, L. et al. Physical health and psychological outcomes in adult patients with long-bone fracture non-unions: evidence today. J. Clin. Med. 8, 1998 (2019).
Kanakaris, N. K. & Giannoudis, P. V. The health economics of the treatment of long-bone non-unions. Injury 38 (Suppl. 2), S77–S84 (2007).
Zeckey, C. et al. The aseptic femoral and tibial shaft non-union in healthy patients - an analysis of the health-related quality of life and the socioeconomic outcome. Open. Orthop. J. 5, 193–197 (2011).
Antonova, E., Le, T. K., Burge, R. & Mershon, J. Tibia shaft fractures: costly burden of nonunions. BMC Musculoskelet. Disord. 14, 42 (2013).
Tay, W. H., de Steiger, R., Richardson, M., Gruen, R. & Balogh, Z. J. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury 45, 1653–1658 (2014).
Wichlas, F. et al. Long-term functional outcome and quality of life after successful surgical treatment of tibial nonunions. Int. Orthop. 39, 521–525 (2015).
Stewart, S. K. Fracture non-union: a review of clinical challenges and future research needs. Malays. Orthop. J. 13, 1–10 (2019).
Lian, J. B. et al. MicroRNA control of bone formation and homeostasis. Nat. Rev. Endocrinol. 8, 212–227 (2012).
Garmilla-Ezquerra, P. et al. Analysis of the bone microRNome in osteoporotic fractures. Calcif. Tissue Int. 96, 30–37 (2015).
Weilner, S. et al. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone 79, 43–51 (2015).
Murata, K. et al. Inhibition of miR-92a enhances fracture healing via promoting angiogenesis in a model of stabilized fracture in young mice. J. Bone Min. Res. 29, 316–326 (2014).
Weilner, S. et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell 15, 744–754 (2016).
Ernst, M., Richards, R. G. & Windolf, M. Smart implants in fracture care - only buzzword or real opportunity? Injury 52 (Suppl. 2), S101–S105 (2021).
Ernst, M. et al. Clinical feasibility of fracture healing assessment through continuous monitoring of implant load. J. Biomech. 116, 110188 (2021).
Li, Z. et al. Pro-osteogenic effects of WNT in a mouse model of bone formation around femoral implants. Calcif. Tissue Int. 108, 240–251 (2021).
Salmon, B. et al. WNT-activated bone grafts repair osteonecrotic lesions in aged animals. Sci. Rep. 7, 14254 (2017).
Burska, A. N. et al. Dynamics of early signalling events during fracture healing and potential serum biomarkers of fracture non-union in humans. J. Clin. Med. 9, 492 (2020).
Kaiser, K. et al. Pharmacological inhibition of IL-6 trans-signaling improves compromised fracture healing after severe trauma. Naunyn Schmiedebergs Arch. Pharmacol. 391, 523–536 (2018).
Dai, Y. et al. Differentially expressed microRNAs as diagnostic biomarkers for infected tibial non-union. Injury 52, 11–18 (2021).
Cheah, E., Wu, Z., Thakur, S. S., O’Carroll, S. J. & Svirskis, D. Externally triggered release of growth factors - a tissue regeneration approach. J. Control. Rel. 332, 74–95 (2021).
Rämö, L. et al. Effect of surgery vs functional bracing on functional outcome among patients with closed displaced humeral shaft fractures: the FISH randomized clinical trial. JAMA 323, 1792–1801 (2020).
Ding, L., He, Z., Xiao, H., Chai, L. & Xue, F. Factors affecting the incidence of aseptic nonunion after surgical fixation of humeral diaphyseal fracture. J. Orthop. Sci. 19, 973–977 (2014).
Ekholm, R., Tidermark, J., Törnkvist, H., Adami, J. & Ponzer, S. Outcome after closed functional treatment of humeral shaft fractures. J. Orthop. Trauma 20, 591–596 (2006).
Thakore, R. V. et al. The Gustilo–Anderson classification system as predictor of nonunion and infection in open tibia fractures. Eur. J. Trauma Emerg. Surg. 43, 651–656 (2017).
Dailey, H. L., Wu, K. A., Wu, P.-S., McQueen, M. M. & Court-Brown, C. M. Tibial fracture nonunion and time to healing after reamed intramedullary nailing: risk factors based on a single-center review of 1003 patients. J. Orthop. Trauma 32, e263–e269 (2018).
Lian, H. & Huang, J. Effectiveness comparison of different operative methods in treatment of closed fracture of tibial shaft. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 29, 1067–1071 (2015).
Gaebler, C., McQueen, M. M., Vécsei, V. & Court-Brown, C. M. Reamed versus minimally reamed nailing: a prospectively randomised study of 100 patients with closed fractures of the tibia. Injury 42, S17–S21 (2011).
Ostrum, R. F., DiCicco, J., Lakatos, R. & Poka, A. Retrograde intramedullary nailing of femoral diaphyseal fractures. J. Orthop. Trauma 12, 464–468 (1998).
Löffler, J. et al. Compromised bone healing in aged rats is associated with impaired M2 macrophage function. Front. Immunol. 10, 2443 (2019).
Waki, T. et al. Profiling microRNA expression in fracture nonunions: potential role of microRNAs in nonunion formation studied in a rat model. Bone Joint J. 97-B, 1144–1151 (2015).
Meesters, D. M. et al. Deficiency of inducible and endothelial nitric oxide synthase results in diminished bone formation and delayed union and nonunion development. Bone 83, 111–118 (2016).
Fajardo, M., Liu, C.-J. & Egol, K. Levels of expression for BMP-7 and several BMP antagonists may play an integral role in a fracture nonunion: a pilot study. Clin. Orthop. Relat. Res. 467, 3071 (2009).
Zimmermann, G. et al. TGF-β1 as a marker of delayed fracture healing. Bone 36, 779–785 (2005).
Tsuji, K. et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 38, 1424–1429 (2006).
Makino, T., Hak, D. J., Hazelwood, S. J., Curtiss, S. & Reddi, A. H. Prevention of atrophic nonunion development by recombinant human bone morphogenetic protein-7. J. Orthop. Res. 23, 632–638 (2005).
Wildemann, B. et al. Local BMP-2 application can rescue the delayed osteotomy healing in a rat model. Injury 42, 746–752 (2011).
Zhang, W. et al. The intracellular NADH level regulates atrophic nonunion pathogenesis through the CtBP2-p300-Runx2 transcriptional complex. Int. J. Biol. Sci. 14, 2023–2036 (2018).
Chen, X. et al. NSM00158 Specifically disrupts the CtBP2-p300 interaction to reverse CtBP2-mediated transrepression and prevent the occurrence of nonunion. Mol. Cell 43, 517–529 (2020).
Baht, G. S. et al. Exposure to a youthful circulation rejuvenates bone repair through modulation of β-catenin. Nat. Commun. 6, 7131 (2015).
Burgers, T. A. et al. Mice with a heterozygous Lrp6 deletion have impaired fracture healing. Bone Res. 4, 16025 (2016).
Liu, Y. et al. Effects of sclerostin antibody on the healing of femoral fractures in ovariectomised rats. Calcif. Tissue Int. 98, 263–274 (2016).
Haffner-Luntzer, M. et al. Inhibition of midkine augments osteoporotic fracture healing. PLoS ONE 11, e0159278 (2016).
McKenzie, J. A. et al. Activation of hedgehog signaling by systemic agonist improves fracture healing in aged mice. J. Orthop. Res. 37, 51–59 (2019).
Weiss, S. et al. Systemic response of the GH/IGF-I axis in timely versus delayed fracture healing. Growth Horm. IGF Res. 18, 205–212 (2008).
Aspenberg, P. et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Min. Res. 25, 404–414 (2010).
Kumabe, Y. et al. Triweekly administration of parathyroid hormone (1–34) accelerates bone healing in a rat refractory fracture model. BMC Musculoskelet. Disord. 18, 545 (2017).
Bostrom, M. P. G. et al. Parathyroid hormone-related protein analog RS-66271 is an effective therapy for impaired bone healing in rabbits on corticosteroid therapy. Bone 26, 437–442 (2000).
Minkwitz, S., Fassbender, M., Kronbach, Z. & Wildemann, B. Longitudinal analysis of osteogenic and angiogenic signaling factors in healing models mimicking atrophic and hypertrophic non-unions in rats. PLoS ONE 10, e0124217 (2015).
Garcia, P. et al. Temporal and spatial vascularization patterns of unions and nonunions: role of vascular endothelial growth factor and bone morphogenetic proteins. J. Bone Joint Surg. Am. 94, 49–58 (2012).
Goel, P. N. et al. Suppression of notch signaling in osteoclasts improves bone regeneration and healing. J. Orthop. Res. 37, 2089–2103 (2019).
Schipani, E., Maes, C., Carmeliet, G. & Semenza, G. L. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J. Bone Min. Res. 24, 1347–1353 (2009).
Acknowledgements
M.J.S. and R.G.R. thank the support from the AO Foundation. A.I. is supported by the Collaborative Research Center CRC1149 (DFG, German Research Foundation; Project number 251293561).
Author information
Authors and Affiliations
Contributions
Introduction (J.B.J.); Epidemiology (F.L.); Mechanisms/pathophysiology (B.W. and A.I.); Diagnosis, screening and prevention (L.A.T. and J.B.J.); Management (R.M.S., M.J.S. and R.G.R.); Quality of life (R.P.); Outlook (J.B.J. and M.J.S.); Overview of Primer (J.B.J. and B.W.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks P. Kloen, P. Leucht, A. Nauth, M. Poeze, S. Rammelt, P. M. Rommens and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Callus
-
Healing tissue that connects the bone ends.
- Sequestrum
-
Necrotic tissue separated from the surrounding tissue.
- Interfragmentary movement
-
Axial compression, tension and shear movements in the callus area.
- Progenitor cells
-
Cells with the potential to differentiate in a more specific cell type.
- Remodelling
-
Physiological process of bone resorption and formation that occurs in mature bone and during healing.
- Intramembranous ossification
-
Direct transformation of mesenchymal tissue into bone.
- Endochondral ossification
-
Bone formation via an intermediate cartilage template.
- Chondrocytes
-
Cells of mesenchymal origin that produce cartilaginous matrix.
- Osteoblasts
-
Bone-forming cells of mesenchymal origin that produce extracellular matrix and are responsible for mineralization.
- Osteoclasts
-
Bone-resorbing cells of haematopoietic origin.
- Osteocytes
-
Terminally differentiated osteoblasts embedded in the mineralized tissue that are important for bone homeostasis.
- Diaphysis
-
Shaft of a long bone, mainly cortical bone.
- Metaphysis
-
Neck portion of a long bone, mainly trabecular bone.
- Primary bone healing
-
Direct healing without an intermediate cartilage template and without callus formation.
- Osteocyte-lacuno-canalicular network
-
Communication network in bone.
- Haversian systems
-
Canals that contain capillaries and nerve fibres, surrounded by concentric layers of mineralized tissue (lamella) forming osteons (substructures of bone tissue).
- Secondary bone healing
-
Indirect healing with callus formation and intramembranous as well as endochondral ossification.
- Macrophages
-
Differentiated monocytes that phagocytize dying and dead cells and cell debris and modulate the inflammatory milieu.
- Healing phases
-
Fracture, haematoma formation, inflammation, repair with soft and hard callus formation, remodelling.
- Angiogenesis
-
Formation of new blood vessels from existing vessels.
- Soft callus
-
Fibrous and cartilaginous tissue.
- Interfragmentary strain
-
Axial interfragmentary movement divided by the fracture gap size.
- Periosteum
-
Cell-rich membrane covering the outer bone surface, important for bone healing.
- Monocytes
-
Type of white blood cell, part of the innate immune system.
- Cortical bone
-
Dense structure that forms the outer shell of long bones.
- Trabecular bone
-
Porous bone formed from an interconnective network of rods and plates that are alined along the lines of stress.
- Delayed union
-
Delay in healing of a fractured bone within the expected time.
- Primary cilia
-
Immotile tubules that project from the cell surface like antennas.
- Hard callus
-
Mineralized tissue.
- Pseudoarthrosis
-
False joint, special form of non-union.
- Osteonecrosis
-
Avascular death (necrosis) of bone.
- Deformity
-
A bone without normal shape or size owing to congenital, developmental or post-traumatic reasons.
- Judet decortication
-
Part of the surgical procedure and local approach to non-unions in which the surface of the local bone is peeled up in thin flakes with an osteotome in continuity with overlying soft tissue to enhance the bleeding area and, therefore, bone formation.
- Critical-size defect
-
Substantial bone loss that does not heal spontaneously despite surgical stabilization.
- Ilizarov technique
-
A surgical procedure based on the application of a ring external fixator to the bone, which can provide mechanical support together with the ability to progressively change the position of the rings and bone to move bone in space.
Rights and permissions
About this article
Cite this article
Wildemann, B., Ignatius, A., Leung, F. et al. Non-union bone fractures. Nat Rev Dis Primers 7, 57 (2021). https://doi.org/10.1038/s41572-021-00289-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-021-00289-8
This article is cited by
-
Fibroblasts inhibit osteogenesis by regulating nuclear-cytoplasmic shuttling of YAP in mesenchymal stem cells and secreting DKK1
Biological Research (2024)
-
Bioinformatics Analysis and Experimental Validation of Differential Genes and Pathways in Bone Nonunions
Biochemical Genetics (2024)
-
A distinct “repair” role of regulatory T cells in fracture healing
Frontiers of Medicine (2024)
-
Bone tissue engineering for osteointegration: Where are we now?
Polymer Bulletin (2024)
-
Deciphering the protective effect of Buzhong Yiqi Decoction on osteoporotic fracture through network pharmacology and experimental validation
Journal of Orthopaedic Surgery and Research (2023)