Abstract
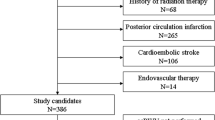
Acute ischemic stroke (IS) is one of the leading causes of morbidity, functional disability and mortality worldwide. The objective was to evaluate IS risk factors and imaging variables as predictors of short-term disability and mortality in IS. Consecutive 106 IS patients were enrolled. We examined the accuracy of IS severity using the National Institutes of Health Stroke Scale (NIHSS), carotid intima-media thickness (cIMT) and carotid stenosis (both assessed using ultrasonography with doppler) predicting IS outcome assessed with the modified Rankin scale (mRS) three months after hospital admission. Poor prognosis (mRS ≥ 3) at three months was predicted by carotid stenosis (≥ 50%), type 2 diabetes mellitus and NIHSS with an accuracy of 85.2% (sensitivity: 90.2%; specificity: 81.8%). The mRS score at three months was strongly predicted by NIHSS (β = 0.709, p < 0.001). Short-term mortality was strongly predicted using a neural network model with cIMT (≥ 1.0 mm versus < 1.0 mm), NIHSS and age, yielding an area under the receiving operator characteristic curve of 0.977 and an accuracy of 94.7% (sensitivity: 100.0%; specificity: 90.9%). High NIHSS (≥ 15) and cIMT (≥ 1.0 mm) increased the probability of dying with hazard ratios of 7.62 and 3.23, respectively. Baseline NIHSS was significantly predicted by the combined effects of age, large artery atherosclerosis stroke, sex, cIMT, body mass index, and smoking. In conclusion, high values of cIMT and NIHSS at admission strongly predict short-term functional impairment as well as mortality three months after IS, underscoring the importance of those measurements to predict clinical IS outcome.





Similar content being viewed by others
References
Albanese MA, Clarke WR, Adams HP, Woolson RF (1994) Ensuring reliability of outcome measures in multicenter clinical trials of treatments for acute ischemic stroke. The program developed for the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Stroke 25:1746–1751
Adams HP Jr, Bendixen BH, Kappelle JJ et al (1993) Classification of subtype of acute ischemic stroke. Definitions of use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41. https://doi.org/10.1161/01.str.24.1.35
Adams HP Jr, Davis PH, Leira EC et al (1999) Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 53:126–131. https://doi.org/10.1212/wnl.53.1.126
Adams HP Jr, del Zoppo G, Alberts MJ et al (2007) Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38:1655–1711. https://doi.org/10.1161/STROKEAHA.107.181486
Alfieri DF, Lehmann MF, Oliveira SR et al (2017) (2017) Vitamin D deficiency is associated with acute ischemic stroke, C-reactive protein, and short-term outcome. Metab Brain Dis 32:493–502. https://doi.org/10.1007/s11011-016-9939-2
Alfieri DF, Lehmann MF, Flauzino T et al (2020) Immune-inflammatory, metabolic, oxidative, and nitrosative stress biomarkers predict acute ischemic stroke and short-term outcome. Neurotox Res 38(2):330–343. https://doi.org/10.1007/s12640-020-00221-0
American Diabetes Association (2016) Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers (2016). Clin Diabetes 34(1):3–21. https://doi.org/10.2337/diaclin.34.1.3
Babu MS, Kaul S, Dadheech S, Rajeshwar K, Jyothy A, Munshi A (2013) Serum albumin levels in ischemic stroke and its subtypes: correlation with clinical outcome. Nutrition 29:872–875
Baggio JA, Santos-Pontelli TE, Cougo-Pinto PT et al (2014) Validation of a structured interview for telephone assessment of the modified Rankin Scale in Brazilian stroke patients. Cerebrovasc Dis 38:297–301. https://doi.org/10.1159/000367646
Benjamin EJ, Virani SS, Callaway CW et al (2018) Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137(12):67–492. https://doi.org/10.1161/CIR.0000000000000558
Bonita R, Beaglehole R (1988) Recovery of motor function after stroke. Stroke 19(12):1497–1500. https://doi.org/10.1161/01.str.19.12.1497
Brazil. (2013) Guidelines for the rehabilitation of individual with cerebrovascular accident. Editor: Ministry of Health. Brasília; Ministry of Health
Brott T, Adams HP Jr, Olinger CP (1989) Measurement of acute cerebral infarction: a clinical examinations scale. Stroke 20:864–870. https://doi.org/10.1161/01.str.20.7.864
Bustamante A, Simats A, Vilar-Bergua A, García-Berrocoso T, Montaner J (2016) Blood/brain biomarkers of inflammation after stroke and their association with outcome: from c-reactive protein to damage-associated molecular patterns. Neurotherapeutics 13(4):671–684. https://doi.org/10.1007/s13311-016-0470-2
Candelise L, Pinardi G, Aritzu E, Musicco M (1994) Telephone interview for stroke outcome assessment. Cerebrovasc Dis 4:341–343
Carvalho JJ, Alves MB, Viana GÁ et al (2011) Stroke epidemiology, patterns of management, and outcomes in Fortaleza, Brazil: a hospital-based multicenter prospective study. Stroke 42(12):3341–3346. https://doi.org/10.1161/STROKEAHA.111.626523
Casas-Vara A, Santolaria F, Fernández-Bereciartúa A, González-Reimers E, García-Ochoa A, Martínez-Riera A (2012) The obesity paradox in elderly patients with heart failure: analysis of nutritional status. Nutrition 28(6):616–622. https://doi.org/10.1016/j.nut.2011.10.006
Chang KC, Lee HC, Tseng MC, Huang YC (2010) Three-year survival after first-ever ischemic stroke is predicted by initial stroke severity: a hospital-based study. Clin Neurol Neurosurg 112(4):296–301. https://doi.org/10.1016/j.clineuro.2009.12.016
Chen X, Zhan X, Chen M et al (2012) The prognostic value of combined NT-pro-BNP levels and NIHSS scores in patients with acute ischemic stroke. Intern Med 51:2887–2892. https://doi.org/10.2169/internalmedicine.51.8027
Cincura C, Pontes-Neto OM, Neville IS et al (2009) Validation of the National Institutes of Health Stroke Scale, modified Rankin Scale and Barthel Index in Brazil: the role of cultural adaptation and structured interviewing. Cerebrovasc Dis 27:119–122. https://doi.org/10.1159/000177918
Costa MACD, Nadal JP, Okamoto JM et al (2019) Prevalence of carotid stenosis and incidence of ischemic stroke in patients undergoing non-coronary cardiac surgery. Braz J Cardiovasc Surg 34:550–559
de Caneda MA, Fernandes JG, de Almeida AG, Mugnol FE (2006) Confiabilidade de escalas de comprometimento neurológico em pacientes com acidente vascular cerebral [Reliability of neurological assessment scales in patients with stroke]. Arq Neuropsiquiatr 64(3A):690–697. https://doi.org/10.1590/s0004-282x2006000400034
Ebrahim S, Papacosta O, Whincup P et al (1999) Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke 30:841–850. https://doi.org/10.1161/01.str.30.4.841
García-Berrocoso T, Giralt D, Bustamante A et al (2013) B-type natriuretic peptides and mortality after stroke: a systematic review and meta-analysis. Neurology 81:1976–1985. https://doi.org/10.1212/01.wnl.0000436937.32410.32
Goldstein LB, Samsa GP (1997) Reliability of the National Institutes of Health Stroke Scale: extension to non-neurologists in the context of a clinical trial. Stroke 28:307–310
Grau AJ, Weimar C, Buggle F et al (2001) Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 32:2559–2566. https://doi.org/10.1161/hs1101.098524
Harpaz D, Eltzov E, Seet RCS, Marks RS, Tok AIY (2017) Point-of-care-testing in acute stroke Management: An unmet need ripe for technological harvest. Biosensors (Basel) 7(3):30. https://doi.org/10.3390/bios7030030
Harpaz D, Seet RCS, Marks RS, Tok AIY (2020) Blood-based biomarkers are associated with different ischemic stroke mechanisms and enable rapid classification between cardioembolic and atherosclerosis etiologies. Diagnostics (basel) 10:804. https://doi.org/10.3390/diagnostics10100804
Heliopoulos I, Papaoiakim M, Tsivgoulis G et al (2009) Common carotid intima media thickness as a marker of clinical severity in patients with symptomatic extracranial carotid artery stenosis. Clin Neurol Neurosurg 111(3):246–250. https://doi.org/10.1016/j.clineuro.2008.10.007
Huang ZX, Huang Y, Zeng J et al (2020) Admission glucose levels may increase the risk for early neurological deterioration in females with acute ischemic stroke. Front Neurol 11:548892. https://doi.org/10.3389/fneur.2020.548892
James PA, Oparil S, Carter BL et al (2014) 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311(5):507–520. https://doi.org/10.1001/jama.2013.284427
Janssen PM, Visser NA, Dorhout Mees SM et al (2010) Comparison of the telephone and face-to-face assessment of the modified Rankin Scale. Cerebrovasc Dis 29:137–139
Jia Q, Liu G, Zheng H et al (2014) Impaired glucose regulation predicted 1-year mortality of Chinese patients with ischemic stroke: data from abnormal glucose regulation in patients with acute stroke across China. Stroke 45(5):1498–1500. https://doi.org/10.1161/STROKEAHA.113.002977
Johnston KC, Connors AF Jr, Wagner DP, Haley EC Jr (2003) Predicting outcome in ischemic stroke: external validation of predictive risk models. Stroke 34:200–202. https://doi.org/10.1161/01.str.0000047102.61863.e3
Johnston KC, Wagner DP, Haley EC Jr, Connor AF Jr (2002) Combined clinical and imaging information as an early stroke outcome measure. Stroke 33(2):466–472. https://doi.org/10.1161/hs0202.102881
Kawai T, Ohishi M, Takeya Y et al (2013) Carotid plaque score and intima media thickness as predictors of stroke and mortality in hypertensive patients. Hypertens Res 36(10):902–909. https://doi.org/10.1038/hr.2013.61
Kim J, Thayabaranathan T, Donnan GA et al (2020) Global Stroke Statistics 2019. Int J Stroke. https://doi.org/10.1177/1747493020909545
Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU (2001) Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 32(12):2735–2740. https://doi.org/10.1161/hs1201.100209
Koton S, Tanne D, Green MS, Bornstein NM (2010) Mortality and predictors of death 1 month and 3 years after first-ever ischemic stroke: data from the First National Acute Stroke Israeli Survey (NASIS 2004). Neuroepidemiology 34(2):90–96. https://doi.org/10.1159/000264826
Kuster GW, Dutra LA, Brasil IP et al (2016) Performance of four ischemic stroke prognostic scores in a Brazilian population. Arq Neuropsiquiatr 74(2):133–137. https://doi.org/10.1590/0004-282X20160002
Laskowitz DT, Kasner SE, Saver J et al (2009) Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke 40:77–85. https://doi.org/10.1161/STROKEAHA.108.516377
Lehmann MF, Kallaur AP, Oliveira SR et al (2015) Inflammatory and metabolic markers and short-time outcome in patients with acute ischemic stroke in relation to TOAST subtypes. Metab Brain Dis 30:1417–1428. https://doi.org/10.1007/s11011-015-9731-8
Licata G, Tuttolomondo A, Corrao S, Di Raimondo D, Fernandez P, Caruso C, Avellone G, Pinto A (2006) Immunoinflammatory activation during the acute phase of lacunar and non-lacunar ischemic stroke: association with time of onset and diabetic state. Int J Immunopathol Pharmacol 19(3):639–646. https://doi.org/10.1177/039463200601900320
Lodder J, Bamford JM, Sandercock PA, Jones LN, Warlow CP (1990) Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke 21:375–381. https://doi.org/10.1161/01.str.21.3.375
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M (2007) Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115:459–467. https://doi.org/10.1161/CIRCULATIONAHA.106.628875
Lyden P, Brott T, Tilley B et al (1994) Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group Stroke 25:2220–2226. https://doi.org/10.1161/01.str.25.11.2220
Lyden P, Raman R, Liu L et al (2005) NIHSS training and certification using a new digital video disk is reliable. Stroke 36:2446–2449. https://doi.org/10.1161/01.STR.0000185725.42768.92
Lyden P (2017) Using the National Institutes of Health Stroke Scale A Cautionary Tale. Stroke 48:513–519. https://doi.org/10.1161/STROKEAHA.116.015434
Lyden P, Raman R, Liu L, Emr M, Warren M, Marler J (2009) National Institutes of Health Stroke Scale certification is reliable across multiple venues. Stroke 4:2507–2511. https://doi.org/10.1161/STROKEAHA.108.532069
Makris K, Haliassos A, Chondrogianni M, Tsivgoulis G (2018) Blood biomarkers in ischemic stroke: potential role and challenges in clinical practice and research. Crit Rev Clin Lab Sci 55(5):294–328. https://doi.org/10.1080/10408363.2018.1461190
Marsh EB, Lawrence E, Gottesman RF, Llinas RH (2016) The NIH Stroke Scale has limited utility in accurate daily monitoring of neurologic status. Neurohospitalist 6:97–101. https://doi.org/10.1177/1941874415619964
Montaner J, García-Berrocoso T, Mendioroz M et al (2012) Brain natriuretic peptide is associated with worsening and mortality in acute stroke patients but adds no prognostic value to clinical predictors of outcome. Cerebrovasc Dis 34:240–245. https://doi.org/10.1159/000341858
Nambi V, Chambless L, Folsom AR et al (2010) Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol 55:1600–1607. https://doi.org/10.1016/j.jacc.2009.11.075
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report (2012) Circulation 106: 3143–3412
Newcommon NJ, Green TL, Haley E et al (2003) Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke 34:377–378
O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr (1999) Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 340:14–22. https://doi.org/10.1056/NEJM199901073400103
Ozkan AK, Yemisci OU, Saracgil Cosar SN, Oztop P, Turhan N (2013) Can high-sensitivity C-reactive protein and ferritin predict functional outcome in acute ischemic stroke? A prospective study. Top Stroke Rehabil 20(6):528–536. https://doi.org/10.1310/tsr2006-528
Park KY, Chung PW, Kim YB et al (2015) Serum vitamin D status as a predictor of prognosis in patients with acute ischemic stroke. Cerebrovasc Dis 40:73–80. https://doi.org/10.1159/000434691
Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO (1999) Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke 30:2513–2516. https://doi.org/10.1161/01.str.30.12.2513
Rangaraju S, Jovin TG, Frankel M et al (2016) Neurologic examination at 24 to 48 hours predicts functional outcomes in basilar artery occlusion stroke. Stroke 47(10):2534–2540. https://doi.org/10.1161/STROKEAHA.116.014567
Reiche EMV, Gelinksi JR, Alfieri DF et al (2019) Immune-inflammatory, oxidative stress and biochemical biomarkers predict short-term acute ischemic stroke death. Metab Brain Dis 34(3):789–804. https://doi.org/10.1007/s11011-019-00403-6
Reynolds MA, Kirchick HJ, Dahlen JR, Anderberg JM, McPherson PH, Nakamura KK, Laskowitz DT, Valkirs GE, Buechler KF (2003) Early biomarkers of stroke. Clin Chem 49(10):1733–1739. https://doi.org/10.1373/49.10.1733
Ringle CM, da Silva D, Bido D (2014) Structural equation modeling with the SmartPLS. Brazilian Journal of Marketing 13(2):18
Saenger AK, Christenson RH (2010) Stroke biomarkers: progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin Chem 56:21–33. https://doi.org/10.1373/clinchem.2009.133801
Santos EHS, Santos Neto PJ, Santos IDS (2018) Carotid intima-media thickness in the Brazilian longitudinal study of adult health (ELSA-Brazil): a narrative review. São Paulo Med J 136(1):64–72. https://doi.org/10.1590/1516-3180.2017.0272141017
Saver JL, Johnston KC, Homer D et al (1999) Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials The RANTTAS Investigators. Stroke 30:293–298. https://doi.org/10.1161/01.str.30.2.293
Savio K, Pietra GL, Oddone E, Reggiani M, Leone MA (2013) Reliability of the modified Rankin Scale applied by telephone. Neurol Int 5:e2. https://doi.org/10.4081/ni.2013.e2
Sharma R, Macy S, Richardson K, Lokhnygina Y, Laskowitz DT (2014) A blood-based biomarker panel to detect acute stroke. J Stroke Cerebrovasc Dis 23(5):910–918. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.07.034
Sharma A, Tate M, Mathew G et al (2018) Oxidative stress and NLRP3-inflammasome activity as significant drivers of diabetic cardiovascular complications: therapeutic implications. Front Physiol 9:114. https://doi.org/10.3389/fphys.2018.00114
Shibazaki K, Kimura K, Sakai K, Fujii S, Aoki J, Saji N (2013) Brain natriuretic peptide on admission as a biological marker of long-term mortality in ischemic stroke survivors. Eur Neurol 70:218–224. https://doi.org/10.1159/000351777
Simão ANC, Lehmann MF, Alfieri DF et al (2015) Metabolic syndrome increases oxidative stress but does not influence disability and short-time outcome in acute ischemic stroke patients. Metab Brain Dis 30:1409–1416. https://doi.org/10.1007/s11011-015-9720-y
Sucharew H, Khoury J, Moomaw CJ et al (2013) Profiles of the National Institutes of Health Stroke Scale items as a predictor of patient outcome. Stroke 44:2182–2187. https://doi.org/10.1161/STROKEAHA.113.001255
Sun W, Huang Y, Xian Y et al (2017) Association of body mass index with mortality and functional outcome after acute ischemic stroke. Sci Rep 7(1):2507. https://doi.org/10.1038/s41598-017-02551-0
Sung JY, Chen CI, Hsieh YC et al (2017) Comparison of admission random glucose, fasting glucose, and glycated hemoglobin in predicting the neurological outcome of acute ischemic stroke: a retrospective study. PeerJ 5:e2948. https://doi.org/10.7717/peerj.2948
Tomlinson DR, Gardiner NJ (2008) Glucose neurotoxicity. Nat Rev Neurosci 9(1):36–45. https://doi.org/10.1038/nrn2294
Touboul PJ, Hennerici MG, Meairs S et al (2004) Mannheim intima-media thickness consensus (2004–2006). Cerebrovasc Dis 18:346–349. https://doi.org/10.1159/000097034
Tziomalos K, Spanou M, Bouziana SD et al (2014) Type 2 diabetes is associated with a worse functional outcome of ischemic stroke. World J Diabetes 5(6):939–944. https://doi.org/10.4239/wjd.v5.i6.939
Tziomalos K, Bouziana SD, Spanou M, Kostaki S, Papadopoulou M, Giampatzis V (2015) Prior treatment with dipeptidyl peptidase 4 inhibitors is associated with better functional outcome and lower in-hospital mortality in patients with type 2 diabetes mellitus admitted with acute ischaemic stroke. Diab Vasc Dis Res 12(6):463–466. https://doi.org/10.1177/1479164115597867
Uchino K, Billheimer D, Cramer SC (2001) Entry criteria and baseline characteristics predict outcome in acute stroke trials. Stroke 32(4):909–916. https://doi.org/10.1161/01.str.32.4.909
Vernino S, Brown RD Jr, Sejvar JJ, Sicks JD, Petty GW, O’Fallon WM (2003) Cause specific mortality after first cerebral infarction: a population-based study. Stroke 34:1828–1832. https://doi.org/10.1161/01.STR.0000080534.98416.A0
Vlachopoulos C, Xaplanteris P, Aboyans V et al (2015) The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 241:507–532. https://doi.org/10.1016/j.atherosclerosis.2015.05.007
Wei W, Li S, San F et al (2018) Retrospective analysis of prognosis and risk factors of patients with stroke by TOAST. Medicine 97(15):e0412. https://doi.org/10.1097/MD.0000000000010412
Weimar C, König IR, Kraywinkel K, Ziegler A, Diener HC; German Stroke Study Collaboration (2004) Age and national institutes of health stroke scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 35(1):158–62. https://doi.org/10.1161/01.STR.0000106761.94985.8B
Welsh P, Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ (2009) Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis 27(3):247–253. https://doi.org/10.1159/000196823
Zhang Y, Fang X, Hua Y et al (2018) Carotid artery plaques, carotid intima-media thickness, and risk of cardiovascular events and all-cause death in older adults: a 5-year prospective. Commun Based Study Angiol 69(2):120–129. https://doi.org/10.1177/0003319717716842
Funding
The study was supported by grants from Fundação Araucária, Paraná State, Brazil, Conv. 001/2017, Call nº 09/2016, Protocol 47.396—Institutional Program of Basic and Applied Research; grants from Coordination for the Improvement of Higher Level of Education Personnel (CAPES) of Brazilian Ministry of Education: Finance Code 001; Institutional Program for Scientific Initiation Scholarship (PIBIC) of the National Council for Scientific and Technological Development (CNPq) of Brazil.
Author information
Authors and Affiliations
Contributions
(1) Conception and design of the study: ANCS, EMVR; (2) acquisition of data: ALCFL, DFA, MCMA, ERT, MRN, FSP, JRG, LBF, TF, MFL, MABL, JWB; (3) statistical analysis: DFA, MM; (4) analysis and interpretation of data: ALCFL, DFA, MM, ANCS, EMVR; (5) drafting of the manuscript, tables and figures: ALCFL, ANCS, DFA, EMVR, MM; (6) manuscript review: EMVR, MM.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare. None of the authors are involved in the publication process or have a financial or other beneficial interest in the products or concepts mentioned in the submitted manuscript.
Ethical approval
The protocol was approved by the Institutional Research Ethics Committees of University of Londrina, Paraná, Brazil (CAAE: (CAAE 61361416.9.0000.5231) and all of the individuals invited were informed in detail about the research and gave written Informed Consent.
Human rights
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institution and/or National Research Committee and with the World Medical Association 1964 Helsinki Declaration.
Standards for reporting
The manuscript was prepared taken into account the recommendations of the guidelines hosted by the Strengthening the Reporting of Observational studies in Epidemiology (STROBE). STROBE is used for observational studies (cohort, case–control, or cross-sectional designs) according to the STROBE statement (www.strobe-statement.org).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The studied participants were informed about the present research, and a written consent form was taken from all of them before their enrollment. Moreover, all the authors and co-authors participated and contributed sufficiently in the research, and all of them concur with the submission. The manuscript has been approved by the responsible authorities where the work was carried out. The authors also concur that, if accepted, the manuscript shall not be published elsewhere in the same form in either the same or any other language, without the consent of the Editor-in Chief of Journal of Stroke and Cerebrovascular Diseases.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lehmann, A.L.C.F., Alfieri, D.F., de Araújo, M.C.M. et al. Carotid intima media thickness measurements coupled with stroke severity strongly predict short-term outcome in patients with acute ischemic stroke: a machine learning study. Metab Brain Dis 36, 1747–1761 (2021). https://doi.org/10.1007/s11011-021-00784-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00784-7