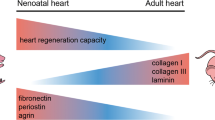
Abstract
Myocardial infarction (MI) occurs due to the obstruction of coronary arteries, a major crux that restricts blood flow and thereby oxygen to the distal part of the myocardium, leading to loss of cardiomyocytes and eventually, if left untreated, leads to heart failure. MI, a potent cardiovascular disorder, requires intense therapeutic interventions and thereby presents towering challenges. Despite the concerted efforts, the treatment strategies for MI are still demanding, which has paved the way for the genesis of biomaterial applications. Biomaterials exhibit immense potentials for cardiac repair and regeneration, wherein they act as extracellular matrix replacing scaffolds or as delivery vehicles for stem cells, protein, plasmids, etc. This review concentrates on natural, synthetic, and hybrid biomaterials; their function; and interaction with the body, mechanisms of repair by which they are able to improve cardiac function in a MI milieu. We also provide focus on future perspectives that need attention. The cognizance provided by the research results certainly indicates that biomaterials could revolutionize the treatment paradigms for MI with a positive impact on clinical translation.
Graphical abstract






Similar content being viewed by others
References
WHO Cardiovascular diseases (CVDs) (2017) https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
Prabhakaran D, Jeemon P, Sharma M et al (2018) The changing patterns of cardiovascular diseases and their risk factors in the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health 6:e1339–e1351. https://doi.org/10.1016/S2214-109X(18)30407-8
Hemalatha T, UmaMaheswari T, Ishwarya S, Gunadharini DN, Anbukkarasi K (2017) Angiogenic factors in myocardial infarction: a critical appraisal. Heart Fail Rev 22:665–683
Creaser JW, DePasquale EC, Vandenbogaart E, Rourke D, Chaker T, Fonarow GC (2015) Team-based care for outpatients with heart failure. Heart Fail Clin 11:379–405
Sun J, Wei T, Bai S, Zhao H, Liu X, Yu J et al (2017) Calcium-sensing receptor-mediated mitogen-activated protein kinase pathway improves the status of transplanted mouse embryonic stem cells in rats with acute myocardial infarction. Mol Cell Biochem 431:151–160. https://doi.org/10.1007/s11010-017-2987-z
Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S et al (2011) Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 78:1847–1857
Rojas SV, Kensah G, Rotaermel A, Baraki H, Kutschka I, Zweigerdt R (2017) Transplantation of purified iPSC-derived cardiomyocytes in myocardial infarction. Plos One 12:e173222. https://doi.org/10.1371/journal.pone.0173222
Rong S, Wang X, Zhang C, Song Z, Cui L, He X et al (2017) Transplantation of HGF gene-engineered skeletal myoblasts improve infarction recovery in a rat myocardial ischemia model. Plos One 12:e175807. https://doi.org/10.1371/journal.pone.0175807
Rabbani S, Soleimani M, Imani M et al (2017) Regenerating heart using a novel compound and human wharton jelly mesenchymal stem cells. Arch Med Res 48:228–237. https://doi.org/10.1016/j.arcmed.2017.03.019
Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C et al (2004) Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364:141–148
Macia E, Boyden PA (2009) Stem cell therapy is proarrhythmic. Circulation 119:1814–1823
Li Y, Wei L, Lan L, Gao Y, Zhang Q, Dawit H, Mao J, Guo L, Shen L, Wang L (2021) Conductive biomaterials for cardiac repair: a review. Acta Biomater S1742–7061(21):00255–00265. https://doi.org/10.1016/j.actbio.2021.04.018
Wu WQ, Peng S, Song ZY, Lin S (2019) Collagen biomaterial for the treatment of myocardial infarction: an update on cardiac tissue engineering and myocardial regeneration. Drug Deliv Transl Res 9:920–934. https://doi.org/10.1007/s13346-019-00627-0
Segers VF, Lee RT (2011) Biomaterials to enhance stem cell function in the heart. Circ Res 109:910–922. https://doi.org/10.1161/CIRCRESAHA.111.249052
Horn MA, Trafford AW (2016) Aging and the cardiac collagen matrix: Novel mediators of fibrotic remodelling. J Mol Cell Cardiol 93:175–185
Lam MT, Wu JC (2012) Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev Cardiovasc Ther 10:1039–1049. https://doi.org/10.1586/erc.12.99
Blackburn NJ, Sofrenovic T, Kuraitis D, Ahmadi A, McNeill B, Deng C et al (2015) Timing underpins the benefits associated with injectable collagen biomaterial therapy for the treatment of myocardial infarction. Biomaterials 39:182–192
Ahmadi A, Thorn SL, Alarcon EI et al (2015) PET imaging of a collagen matrix reveals its effective injection and targeted retention in a mouse model of myocardial infarction. Biomaterials 49:18–26. https://doi.org/10.1016/j.biomaterials.2015.01.016
Serpooshan V, Zhao M, Metzler SA et al (2013) The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials 34:9048–9055. https://doi.org/10.1016/j.biomaterials.2013.08.017
Simpson D, Liu H, Fan THM, Nerem R, Dudley SC (2007) A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells 25:2350–2357. https://doi.org/10.1634/stemcells.2007-0132
Araña M, Gavira JJ, Peña E et al (2014) Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials 35:143–151. https://doi.org/10.1016/j.biomaterials.2013.09.083
Liu Y, Xu Y, Wang Z et al (2016) Electrospun nanofibrous sheets of collagen/elastin/polycaprolactone improve cardiac repair after myocardial infarction. Am J Transl Res 8:1678–1694
Maeda K, Rafatian G, Seymour R, Ruel M, Davis DR, Suuronen EJ (2015) Pre-treating the myocardium with collagen matrix improves the efficacy of cardiac stem cell therapy for myocardial infarction. Can J Cardiol 31:S162. https://doi.org/10.1016/j.cjca.2015.07.348
Munarin F, Kant RJ, Rupert CE, Khoo A, Coulombe KLK (2020) Engineered human myocardium with local release of angiogenic proteins improves vascularization and cardiac function in injured rat hearts. Biomaterials 251:120033. https://doi.org/10.1016/j.biomaterials.2020.120033
Fan C, Shi J, Zhuang Y et al (2019) Myocardial-infarction-responsive smart hydrogels targeting matrix metalloproteinase for on-demand growth factor delivery. Adv Mater 31:e1902900. https://doi.org/10.1002/adma.201902900
Gao J, Liu J, Gao Y, Wang C, Zhao Y, Chen B et al (2011) A myocardial patch made of collagen membranes loaded with collagen-binding human vascular endothelial growth factor accelerates healing of the injured rabbit heart. Tissue Eng A 17:2739–2747. https://doi.org/10.1089/ten.tea.2011.0105
Chachques JC, Trainini JC, Lago N, Cortes- Morichetti M, Schussler O, Carpentier A (2008) Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM trial): clinical feasibility study. Ann Thorac Surg 85:901–908. https://doi.org/10.1016/j.athoracsur.2007.10.052
McLaughlin S, McNeill B, Podrebarac J et al (2019) Injectable human recombinant collagen matrices limit adverse remodeling and improve cardiac function after myocardial infarction. Nat Commun 10:4866. https://doi.org/10.1038/s41467-019-12748-8
Ilamaran M, Janeena A, Valappil S, Ramudu KN, Shanmugam G, Niraikulam A (2019) A self-assembly and higher order structure forming triple helical protein as a novel biomaterial for cell proliferation. Biomater Sci 7:2191–2199. https://doi.org/10.1039/c9bm00186g
Ahmadi A, McNeill B, Vulesevic B et al (2014) The role of integrin α2 in cell and matrix therapy that improves perfusion, viability and function of infarcted myocardium. Biomaterials 35:4749–4758. https://doi.org/10.1016/j.biomaterials.2014.02.028
Ungerleider JL, Christman KL (2014) Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl Med 3:1090–1099. https://doi.org/10.5966/sctm.2014-0049
Barsotti MC, Felice F, Balbarini A, Di Stefano R (2011) Fibrin as a scaffold for cardiac tissue engineering. Biotechnol Appl Biochem 58:301–110. https://doi.org/10.1002/bab.49
Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ (2004) Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng 10:403–409. https://doi.org/10.1089/107632704323061762
Yu J, Christman KL, Chin E, Sievers RE, Saeed M, Lee RJ (2009) Restoration of left ventricular geometry and improvement of left ventricular function in a rodent model of chronic ischemic cardiomyopathy. J Thorac Cardiovasc Surg 137:180–187. https://doi.org/10.1016/j.jtcvs.2008.08.036
Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ (2004) Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol 44:654–660. https://doi.org/10.1016/j.jacc.2004.04.040
Ryu JH, Kim IK, Cho SW et al (2005) Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials 26:319–326
Huang NF, Sievers RE, Park JS, Fang Q, Li S, Lee RJ (2006) A rodent model of myocardial infarction for testing the efficacy of cells and polymers for myocardial reconstruction. Nat Protoc 1:1596–1609. https://doi.org/10.1038/nprot.2006.188
Zhang X, Wang H, Ma X et al (2010) Preservation of the cardiac function in infarcted rat hearts by the transplantation of adipose-derived stem cells with injectable fibrin scaffolds. Exp Biol Med (Maywood) 235:1505–1515. https://doi.org/10.1258/ebm.2010.010175
Gonçalves G, Vassallo P, dos Santos L et al (2010) Intramyocardial transplantation of fibroblasts expressing vascular endothelial growth factor attenuates cardiac dysfunction. Gene Ther 17:305–314. https://doi.org/10.1038/gt.2009.146
Kipshidze N, Chekanov V, Chawla P et al (2000) Angiogenesis in a patient with ischemic limb induced by intramuscular injection of vascular endothelial growth factor and fibrin platform. Tex Heart Inst J 27:196–200
Christman KL, Fang Q, Yee MS, Johnson KR, Sievers RE, Lee RJ (2005) Enhanced neovasculature formation in ischemic myocardium following delivery of pleiotrophin plasmid in a biopolymer. Biomaterials 26:1139–1144. https://doi.org/10.1016/j.biomaterials.2004.04.025
Nie SP, Wang X, Qiao SB et al (2010) Improved myocardial perfusion and cardiac function by controlled-release basic fibroblast growth factor using fibrin glue in a canine infarct model. J Zhejiang Univ Sci B 11:895–904. https://doi.org/10.1631/jzus.B1000302
Goerler H, Oppelt P, Abel U, Haverich A (2007) Safety of the use of Tissucol Duo S in cardiovascular surgery: retrospective analysis of 2149 patients after coronary artery bypass grafting. Eur J Cardiothorac Surg 32:560–566. https://doi.org/10.1016/j.ejcts.2007.01.071
Guo HD, Wang HJ, Tan YZ, Wu JH (2011) Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng Part A 17:45–58. https://doi.org/10.1089/ten.TEA.2010.0124
Terrovitis J, Lautamäki R, Bonios M et al (2009) Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol 54:1619–1626. https://doi.org/10.1016/j.jacc.2009.04.097
Ruvinov E, Cohen S (2016) Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: from ocean algae to patient bedside. Adv Drug Deliv Rev 96:54–76. https://doi.org/10.1016/j.addr.2015.04.021
Sun J, Tan H (2013) Alginate-based biomaterials for regenerative medicine applications. Materials (Basel) 6:1285–1309. https://doi.org/10.3390/ma6041285
Landa N, Miller L, Feinberg MS et al (2008) Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 117:1388–1396. https://doi.org/10.1161/CIRCULATIONAHA.107.727420
Leor J, Tuvia S, Guetta V et al (2009) Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol 54:1014–1023. https://doi.org/10.1016/j.jacc.2009.06.010
Deng B, Shen L, Wu Y, Shen Y, Ding X, Lu S, Jia J, Qian J, Ge J (2015) Delivery of alginate-chitosan hydrogel promotes endogenous repair and preserves cardiac function in rats with myocardial infarction. J Biomed Mater Res Part A 103A:907–918
Rocca DG, Willenberg BJ, Qi Y et al (2016) An injectable capillary-like microstructured alginate hydrogel improves left ventricular function after myocardial infarction in rats. Int J Cardiol 220:149–154. https://doi.org/10.1016/j.ijcard.2016.06.158
Hao T, Li J, Yao F et al (2017) Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano 11:5474–5488. https://doi.org/10.1021/acsnano.7b00221
Karpov AA, Puzanov MV, Ivkin DY et al (2019) Non-inferiority of microencapsulated mesenchymal stem cells to free cells in cardiac repair after myocardial infarction: a rationale for using paracrine factor(s) instead of cells. Int J Exp Pathol 100:102–113. https://doi.org/10.1111/iep.12312
Dvir T, Kedem A, Ruvinov E et al (2009) Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc Natl Acad Sci USA 106:14990–14995. https://doi.org/10.1073/pnas.0812242106
Ruvinov E, Leor J, Cohen S (2011) The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials 32:565–578. https://doi.org/10.1016/j.biomaterials.2010.08.097
Feng J, Wu Y, Chen W, Li J, Wang X, Chen Y, Yu Y, Shen Z, Zhang Y (2020) Sustained release of bioactive IGF-1 from a silk fibroin microsphere-based injectable alginate hydrogel for the treatment of myocardial infarction. J Mater Chem B 8:308–315
Henri O, Pouehe C, Houssari M et al (2016) Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation 133:1484–1497. https://doi.org/10.1161/CIRCULATIONAHA.115.020143
Kontonika M, Barka E, Roumpi M et al (2017) Prolonged intra-myocardial growth hormone administration ameliorates post-infarction electrophysiologic remodeling in rats. Growth Factors 35:1–11. https://doi.org/10.1080/08977194.2017.1297432
Lv K, Li Q, Zhang L et al (2019) Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics 9:7403–7416. https://doi.org/10.7150/thno.32637
Frey N, Linke A, Süselbeck T et al (2014) Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ Cardiovasc Interv 7:806–812. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001478
Rao SV, Zeymer U, Douglas PS et al (2015) A randomized, double-blind, placebo-controlled trial to evaluate the safety and effectiveness of intracoronary application of a novel bioabsorbable cardiac matrix for the prevention of ventricular remodeling after large ST-segment elevation myocardial infarction: Rationale and design of the PRESERVATION I trial. Am Heart J 170:929–937. https://doi.org/10.1016/j.ahj.2015.08.017
Anker SD, Coats AJ, Cristian G et al (2015) A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur Heart J 36:2297–2309. https://doi.org/10.1093/eurheartj/ehv259
Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R, Saldaña-Koppel DA, Quiñones-Olvera LF (2015) Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed Res Int 2015:821279. https://doi.org/10.1155/2015/821279
Ahmadi A, Vulesevic B, Blackburn NJR, Ruel J, Suuronen EJ (2014) A Collagen-chitosan injectable hydrogel improves cardiac remodeling in a mouse model of myocardial infarction. J Biomater Tissue Eng 4:886
Cui Z, Ni NC, Wu J et al (2018) Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation. Theranostics 8:2752–2764. https://doi.org/10.7150/thno.22599
Henning RJ, Khan A, Jimenez E (2016) Chitosan hydrogels significantly limit left ventricular infarction and remodeling and preserve myocardial contractility. J Surg Res 201:490–497. https://doi.org/10.1016/j.jss.2015.11.012
Morimoto Y, Sugimoto T, Haba F, Sakahira H (2016) A new hybrid sutureless patch repair utilizing chitosan for left ventricle rupture after myocardial infarction: a case report. Int J Surg Case Rep 26:131–133. https://doi.org/10.1016/j.ijscr.2016.07.032
Lu WN, Lü SH, Wang HB et al (2009) Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng Part A 15:1437–1447. https://doi.org/10.1089/ten.tea.2008.0143
Wang H, Shi J, Wang Y et al (2014) Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials 35:3986–3998. https://doi.org/10.1016/j.biomaterials.2014.01.021
Xu B, Li Y, Deng B, Liu X, Wang L, Zhu QL (2017) Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats. Exp Ther Med 13:588–594. https://doi.org/10.3892/etm.2017.4026
Chen J, Zhan Y, Wang Y, Han D, Tao B, Luo Z, Ma S, Wang Q, Li X, Fan L, Li C, Deng H, Cao F (2018) Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater 80:154–168. https://doi.org/10.1016/j.actbio.2018.09.013
Wang X, Wang L, Wu Q et al (2019) Chitosan/calcium silicate cardiac patch stimulates cardiomyocyte activity and myocardial performance after infarction by synergistic effect of bioactive ions and aligned nanostructure. ACS Appl Mater Interfaces 11:1449–1468. https://doi.org/10.1021/acsami.8b17754
Henning RJ, Khan A, Wang X (2019) Human umbilical cord stem cells in chitosan attenuate myocardial injury in rat cardiac infarction. Int J Stem Cell Res Ther 6:061. https://doi.org/10.23937/2469-570X/1410061
Liu Y, Li P, Qiao C et al (2020) Chitosan hydrogel enhances the therapeutic efficacy of bone marrow-derived mesenchymal stem cells for myocardial infarction by alleviating vascular endothelial cell pyroptosis. J Cardiovasc Pharmacol 75:75–83. https://doi.org/10.1097/FJC.0000000000000760
Liu Z, Wang H, Wang Y et al (2012) The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials 33:3093–3106. https://doi.org/10.1016/j.biomaterials.2011.12.044
Ishihara M, Nakanishi K, Ono K et al (2002) Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 23:833
Deng C, Zhang P, Vulesevic B et al (2010) A collagene chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng Part A 16:3099
Hughes CS, Postovit LM, Lajoie GA (2010) Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10:1886–1890. https://doi.org/10.1002/pmic.200900758
Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC (2005) Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation 112:I173–I177. https://doi.org/10.1161/CIRCULATIONAHA.104.526178
Ou L, Li W, Zhang Y, Wang W, Liu J, Sorg H et al (2011) Intracardiac injection of matrigel induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model. J Cell Mol Med 15:1310–1318. https://doi.org/10.1111/j.1582-4934.2010.01086.x
Kofidis T, de Bruin JL, Hoyt G et al (2004) Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg 128:571–578. https://doi.org/10.1016/j.jtcvs.2004.05.021
Giraud MN, Ayuni E, Cook S, Siepe M, Carrel TP, Tevaearai HT (2008) Hydrogel-based engineered skeletal muscle grafts normalize heart function early after myocardial infarction. Artif Organs 32:692–700. https://doi.org/10.1111/j.1525-1594.2008.00595.x
Kutschka I, Chen IY, Kofidis T et al (2006) Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation 114:I167–I173. https://doi.org/10.1161/CIRCULATIONAHA.105.001297
Huang G, Huang H (2018) Application of hyaluronic acid as carriers in drug delivery. Drug Deliv 25:766–772. https://doi.org/10.1080/10717544.2018.1450910
Yoon SJ, Fang YH, Lim CH et al (2009) Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Biomed Mater Res B Appl Biomater 91:163–171. https://doi.org/10.1002/jbm.b.31386
Yoon SJ, Hong S, Fang YH et al (2014) Differential regeneration of myocardial infarction depending on the progression of disease and the composition of biomimetic hydrogel. J Biosci Bioeng 118:461–468. https://doi.org/10.1016/j.jbiosc.2014.04.001
Le LV, Mohindra P, Fang Q et al (2018) Injectable hyaluronic acid based microrods provide local micromechanical and biochemical cues to attenuate cardiac fibrosis after myocardial infarction. Biomaterials 169:11–21. https://doi.org/10.1016/j.biomaterials.2018.03.042
Wang N, Liu C, Wang X et al (2019) Hyaluronic acid oligosaccharides improve myocardial function reconstruction and angiogenesis against myocardial infarction by regulation of macrophages. Theranostics 9:1980–1992. https://doi.org/10.7150/thno.31073
Chen CH, Wang SS, Wei EI, Chu TY, Hsieh PC (2013) Hyaluronan enhances bone marrow cell therapy for myocardial repair after infarction. Mol Ther 21:670–679. https://doi.org/10.1038/mt.2012.268
Chang CY, Chan AT, Armstrong PA et al (2012) Hyaluronic acid-human blood hydrogels for stem cell transplantation. Biomaterials 33:8026–8033. https://doi.org/10.1016/j.biomaterials.2012.07.058
Wang W, Tan B, Chen J et al (2018) An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials 160:69–81. https://doi.org/10.1016/j.biomaterials.2018.01.021
Gaffey AC, Chen MH, Trubelja A et al (2019) Delivery of progenitor cells with injectable shear-thinning hydrogel maintains geometry and normalizes strain to stabilize cardiac function after ischemia. J Thorac Cardiovasc Surg 157:1479–1490. https://doi.org/10.1016/j.jtcvs.2018.07.117
Purcell BP, Barlow SC, Perreault PE et al (2018) Delivery of a matrix metalloproteinase-responsive hydrogel releasing TIMP-3 after myocardial infarction: effects on left ventricular remodelling. Am J Physiol Heart Circ Physiol 315:H814–H825. https://doi.org/10.1152/ajpheart.00076.2018
Lee J, Song M, Kim J, Park Y (2018) Comparison of angiogenic activities of three neuropeptides, substance p, secretoneurin, and neuropeptide Y using myocardial infarction. Tissue Eng Regen Med 15:493–502. https://doi.org/10.1007/s13770-018-0134-x
Cheng K, Blusztajn A, Shen D et al (2012) Functional performance of human cardiosphere-derived cells delivered in an in situ polymerizable hyaluronan-gelatin hydrogel. Biomaterials 33:5317–5324. https://doi.org/10.1016/j.biomaterials.2012.04.006
Ogasawara T, Okano S, Ichimura H et al (2017) Impact of extracellular matrix on engraftment and maturation of pluripotent stem cell-derived cardiomyocytes in a rat myocardial infarct model. Sci Rep 7:8630. https://doi.org/10.1038/s41598-017-09217-x
Su K, Wang C (2015) Recent advances in the use of gelatin in biomedical research. Biotechnol Lett 37:2139–2145
Bai X, Fang R, Zhang S, Shi X, Wang Z, Chen X et al (2013) Self-cross-linkable hydrogels composed of partially oxidized alginate and gelatin for myocardial infarction repair. J Bioact Compat Polym 28:126–140. https://doi.org/10.1177/0883911512473230
Walker BW, Lara RP, Yu CH et al (2019) Engineering a naturally-derived adhesive and conductive cardiopatch. Biomaterials 207:89–101. https://doi.org/10.1016/j.biomaterials.2019.03.015
Smith RR, Marbán E, Marbán L (2013) Enhancing retention and efficacy of cardiosphere-derived cells administered after myocardial infarction using a hyaluronan-gelatin hydrogel. Biomatter 3:e24490. https://doi.org/10.4161/biom.24490
Nakajima K, Fujita J, Matsui M et al (2015) Gelatin hydrogel enhances the engraftment of transplanted cardiomyocytes and angiogenesis to ameliorate cardiac function after myocardial infarction. PLoS One 10:e0133308. https://doi.org/10.1371/journal.pone.0133308
Feyen DA, Gaetani R, Deddens J et al (2016) Gelatin microspheres as vehicle for cardiac progenitor cells delivery to the myocardium. Adv Healthc Mater 5:1071–1079. https://doi.org/10.1002/adhm.201500861
Gottipati A, Chelvarajan L, Peng H et al (2019) Gelatin based polymer cell coating improves bone marrow-derived cell retention in the heart after myocardial infarction. Stem Cell Rev Rep 15:404–414. https://doi.org/10.1007/s12015-018-9870-5
Chen Y, Li C, Li C et al (2020) Tailorable hydrogel improves retention and cardioprotection of intramyocardial transplanted mesenchymal stem cells for the treatment of acute myocardial infarction in mice. J Am Heart Assoc 9:e013784. https://doi.org/10.1161/JAHA.119.013784
Li Z, Masumoto H, Jo JI et al (2018) Sustained release of basic fibroblast growth factor using gelatin hydrogel improved left ventricular function through the alteration of collagen subtype in a rat chronic myocardial infarction model. Gen Thorac Cardiovasc Surg 66:641–647. https://doi.org/10.1007/s11748-018-0969-z
Kumagai M, Minakata K, Masumoto H et al (2018) A therapeutic angiogenesis of sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel sheets in a canine chronic myocardial infarction model. Heart Vessels 33:1251–1257. https://doi.org/10.1007/s00380-018-1185-6
Fujita M, Otani H, Iwasaki M et al (2016) Antagomir-92a impregnated gelatin hydrogel microsphere sheet enhances cardiac regeneration after myocardial infarction in rats. Regen Ther 5:9–16. https://doi.org/10.1016/j.reth.2016.04.002
Bejleri D, Davis ME (2019) Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv Healthcare Mater 8:1801217. https://doi.org/10.1002/adhm.201801217
Pomeroy JE, Helfer A, Bursac N (2020) Biomaterializing the promise of cardiac tissue engineering. Biotechnol Adv 42:07353. https://doi.org/10.1016/j.biotechadv.2019.02.009
Perea-Gil I, Prat-Vidal C, Bayes-Genis A (2015) In vivo experience with natural scaffolds for myocardial infarction: the times they are a-changin. Stem Cell Res Ther 6:248. https://doi.org/10.1186/s13287-015-0237-4
Chen WC, Wang Z, Missinato MA et al (2016) Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci Adv 2:e1600844. https://doi.org/10.1126/sciadv.1600844
D’Amore A, Yoshizumi T, Luketich SK et al (2016) Bi-layered polyurethane - Extracellular matrix cardiac patch improves ischemic ventricular wall remodeling in a rat model. Biomaterials 107:1–14. https://doi.org/10.1016/j.biomaterials.2016.07.039
Gálvez-Montón C, Fernandez-Figueras MT, Martí M et al (2015) Neoinnervation and neovascularization of acellular pericardial-derived scaffolds in myocardial infarcts. Stem Cell Res Ther 6:108. https://doi.org/10.1186/s13287-015-0101-6
Efraim Y, Sarig H, Cohen Anavy N et al (2017) Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater 50:220–233. https://doi.org/10.1016/j.actbio.2016.12.015
Perea-Gil I, Prat-Vidal C, Gálvez-Montón C et al (2016) A cell-enriched engineered myocardial graft limits infarct size and improves cardiac function: pre-clinical study in the porcine myocardial infarction model. JACC Basic Transl Sci 1:360–372. https://doi.org/10.1016/j.jacbts.2016.06.005
Perea-Gil I, Gálvez-Montón C, Prat-Vidal C et al (2018) Head-to-head comparison of two engineered cardiac grafts for myocardial repair: from scaffold characterization to pre-clinical testing. Sci Rep 8:6708. https://doi.org/10.1038/s41598-018-25115-2
Jiang Y, Sun SJ, Zhen Z, Wei R, Zhang N, Liao SY, Tse HF (2021) Myocardial repair of bioengineered cardiac patches with decellularized placental scaffold and human-induced pluripotent stem cells in a rat model of myocardial infarction. Stem Cell Res Ther 12:13. https://doi.org/10.1186/s13287-020-02066-y
Mewhort HE, Turnbull JD, Meijndert HC, Ngu JM, Fedak PW (2014) Epicardial infarct repair with basic fibroblast growth factor-enhanced CorMatrix- ECM biomaterial attenuates postischemic cardiac remodelling. J Thorac Cardiovasc Surg 147:1650–1659
Mewhort HE, Turnbull JD, Satriano A et al (2016) Epicardial infarct repair with bioinductive extracellular matrix promotes vasculogenesis and myocardial recovery. J Heart Lung Transplant 35:661–670
Wang Z, Long DW, Huang Y, Chen WCW, Kim K, Wang Y (2019) Decellularized neonatal cardiac extracellular matrix prevents widespread ventricular remodeling in adult mammals after myocardial infarction. Acta Biomater 87:140–151. https://doi.org/10.1016/j.actbio.2019.01.062
Henry JJD, Delrosario L, Fang J et al (2020) Development of injectable amniotic membrane matrix for postmyocardial infarction tissue repair. Adv Healthc Mater 9:e1900544. https://doi.org/10.1002/adhm.201900544
Qiao L, Kong Y, Shi Y et al (2019) Synergistic effects of adipose-derived stem cells combined with decellularized myocardial matrix on the treatment of myocardial infarction in rats. Life Sci 239:116891. https://doi.org/10.1016/j.lfs.2019.116891
Sonnenberg SB, Rane AA, Liu CJ et al (2015) Delivery of an engineered HGF fragment in an extracellular matrix-derived hydrogel prevents negative LV remodeling post-myocardial infarction. Biomaterials 45:56–63. https://doi.org/10.1016/j.biomaterials.2014.12.021
Jang J, Park HJ, Kim SW et al (2017) 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 112:264–274. https://doi.org/10.1016/j.biomaterials.2016.10.026
Traverse JH, Henry TD, Dib N et al (2019) First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl Sci 4:659–669. https://doi.org/10.1016/j.jacbts.2019.07.012
Mewhort HEM, Svystonyuk DA, Turnbull JD et al (2017) Bioactive extracellular matrix scaffold promotes adaptive cardiac remodeling and repair. JACC Basic Transl Sci 2:450–464. https://doi.org/10.1016/j.jacbts.2017.05.005
Sarig U, Sarig H, de- Berardinis E, et al (2016) Natural myocardial ECM patch drives cardiac progenitor based restoration even after scarring. Acta Biomater 44:209–220. https://doi.org/10.1016/j.actbio.2016.08.031
Feroz S, Muhammad N, Ranayake J, Dias G (2020) Keratin-based materials for biomedical applications. Bioact Mater 5:496–509. https://doi.org/10.1016/j.bioactmat.2020.04.007
Shen D, Wang X, Zhang L et al (2011) The amelioration of cardiac dysfunction after myocardial infarction by the injection of keratin biomaterials derived from human hair. Biomaterials 32:9290–9299. https://doi.org/10.1016/j.biomaterials.2011.08.057
Wang T, Jiang XJ, Tang QZ et al (2009) Bone marrow stem cells implantation with alpha-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomater 5:2939–2944. https://doi.org/10.1016/j.actbio.2009.04.040
Wu J, Zeng F, Huang XP et al (2011) Infarct stabilization and cardiac repair with a VEGF-conjugated, injectable hydrogel. Biomaterials 32:579–586. https://doi.org/10.1016/j.biomaterials.2010.08.098
Kraehenbuehl TP, Ferreira LS, Hayward AM et al (2011) Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials 32:1102–1109. https://doi.org/10.1016/j.biomaterials.2010.10.005
Kim DH, Kshitiz SRR et al (2012) Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integr Biol (Camb) 4:1019–1033
Wang H, Liu Z, Li D et al (2012) Injectable biodegradable hydrogels for embryonic stem cell transplantation: improved cardiac remodelling and function of myocardial infarction. J Cell Mol Med 16:1310–1320. https://doi.org/10.1111/j.1582-4934.2011.01409.x
Zhou J, Yang X, Liu W et al (2018) Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct. Theranostics 8:3317–3330. https://doi.org/10.7150/thno.25504
Chang MY, Yang YJ, Chang CH et al (2013) Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. J Control Release 170:287–294. https://doi.org/10.1016/j.jconrel.2013.04.022
Guex AG, Frobert A, Valentin J et al (2014) Plasma-functionalized electrospun matrix for biograft development and cardiac function stabilization. Acta Biomater 10:2996–3006. https://doi.org/10.1016/j.actbio.2014.01.006
Kang BJ, Kim H, Lee SK et al (2014) Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater 10:3007–3017. https://doi.org/10.1016/j.actbio.2014.03.013
Li X, Zhou J, Liu Z et al (2014) A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 35:5679–5688. https://doi.org/10.1016/j.biomaterials.2014.03.067
Zhu H, Jiang X, Li X et al (2016) Intramyocardial delivery of VEGF165 via a novel biodegradable hydrogel induces angiogenesis and improves cardiac function after rat myocardial infarction. Heart Vessels 31:963–975. https://doi.org/10.1007/s00380-015-0710-0
Zhu H, Li X, Yuan M et al (2017) Intramyocardial delivery of bFGF with a biodegradable and thermosensitive hydrogel improves angiogenesis and cardio-protection in infarcted myocardium. Exp Ther Med 14:3609–3615. https://doi.org/10.3892/etm.2017.5015
Matsumura Y, Zhu Y, Jiang H et al (2019) Intramyocardial injection of a fully synthetic hydrogel attenuates left ventricular remodeling post myocardial infarction. Biomaterials 217:119289. https://doi.org/10.1016/j.biomaterials.2019.119289
Wen Y, Li XY, Li ZY et al (2020) Intra-myocardial delivery of a novel thermosensitive hydrogel inhibits post-infarct heart failure after degradation in rat. J Cardiovasc Transl Res 3:677–685. https://doi.org/10.1007/s12265-019-09941-x
Yang Y, Lei D, Huang S et al (2019) Elastic 3d-printed hybrid polymeric scaffold improves cardiac remodeling after myocardial infarction. Adv Healthc Mater 8:e1900065. https://doi.org/10.1002/adhm.201900065
Castellano D, Blanes M, Marco B et al (2014) A comparison of electrospun polymers reveals poly(3-hydroxybutyrate) fiber as a superior scaffold for cardiac repair. Stem Cells Dev 23:1479–1490. https://doi.org/10.1089/scd.2013.0578
Yao Y, Ding J, Wang Z et al (2020) ROS-responsive polyurethane fibrous patches loaded with methylprednisolone (MP) for restoring structures and functions of infarcted myocardium in vivo. Biomaterials 232:119726. https://doi.org/10.1016/j.biomaterials.2019.119726
Fathi E, Nassiri SM, Atyabi N et al (2013) Induction of angiogenesis via topical delivery of basic-fibroblast growth factor from polyvinyl alcohol-dextran blend hydrogel in an ovine model of acute myocardial infarction. J Tissue Eng Regen Med 7:697–707. https://doi.org/10.1002/term.1460
Cai H, Wu FY, Wang QL et al (2019) Self-assembling peptide modified with QHREDGS as a novel delivery system for mesenchymal stem cell transplantation after myocardial infarction. FASEB J 33:8306–8320. https://doi.org/10.1096/fj.201801768RR
Chachques JC, Lila N, Soler-Botija C, Martinez-Ramos C, Valles A, Autret G et al (2020) Elastomeric cardiopatch scaffold for myocardial repair and ventricular support. Eur J Cardiothorac Surg 57:545–555. https://doi.org/10.1093/ejcts/ezz252
Firoozi S, Pahlavan S, Ghanian MH et al (2020) A Cell-Free SDKP-conjugated self-assembling peptide hydrogel sufficient for improvement of myocardial infarction. Biomolecules 10:205. https://doi.org/10.3390/biom10020205
Wang YL, Yu SN, Shen HR, Wang HJ, Wu XP, Wang QL, Zhou B, Tan YZ (2021) Thymosin β4 released from functionalized self-assembling peptide activates epicardium and enhances repair of infarcted myocardium. Theranostics 11:4262–4280. https://doi.org/10.7150/thno.52309
Shapira A, Feiner R, Dvir T (2016) Composite biomaterial scaffolds for cardiac tissue engineering. Int Mater Rev 61:1–19
Noshadi I, Hong S, Sullivan KE et al (2017) In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater Sci 5:2093–2105. https://doi.org/10.1039/c7bm00110j
He Y, Ye G, Song C et al (2018) Mussel-inspired conductive nanofibrous membranes repair myocardial infarction by enhancing cardiac function and revascularization. Theranostics 8:5159–5177. https://doi.org/10.7150/thno.27760
Ye G, Wen Z, Wen F et al (2020) Mussel-inspired conductive Ti2C-cryogel promotes functional maturation of cardiomyocytes and enhances repair of myocardial infarction. Theranostics 10:2047–2066. https://doi.org/10.7150/thno.38876
Xu G, Wang X, Deng C et al (2015) Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)-poly(ethylene glycol)-oligo(acryloyl carbonate) copolymer for functional cardiac regeneration. Acta Biomater 15:55–64. https://doi.org/10.1016/j.actbio.2014.12.016
Zhang Y, Zhu D, Wei Y et al (2019) A collagen hydrogel loaded with HDAC7-derived peptide promotes the regeneration of infarcted myocardium with functional improvement in a rodent model. Acta Biomater 86:223–234. https://doi.org/10.1016/j.actbio.2019.01.022
Bao R, Tan B, Liang S, Zhang N, Wang W, Liu W (2017) A π-π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterials 122:63–71. https://doi.org/10.1016/j.biomaterials.2017.01.012
Rabbani S, Soleimani M, Sahebjam M et al (2018) Simultaneous delivery of wharton’s jelly mesenchymal stem cells and insulin-like growth factor-1 in acute myocardial infarction. Iran J Pharm Res 17:426–441
Shu Y, Hao T, Yao F, Qian Y, Wang Y, Yang B, Li J, Wang C (2015) RoY peptide-modified chitosan-based hydrogel to improve angiogenesis and cardiac repair under hypoxia. ACS Appl Mater Interfaces 7:6505–6517
Liang W, Chen J, Li L et al (2019) Conductive hydrogen sulfide-releasing hydrogel encapsulating adscs for myocardial infarction treatment. ACS Appl Mater Interfaces 11:14619–14629. https://doi.org/10.1021/acsami.9b01886
Wang QL, Wang HJ, Li ZH, Wang YL, Wu XP, Tan YZ (2017) Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. J Cell Mol Med 21:1751–1766. https://doi.org/10.1111/jcmm.13097
Waters R, Alam P, Pacelli S, Chakravarti AR, Ahmed RPH, Paul A (2018) Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater 69:95–106. https://doi.org/10.1016/j.actbio.2017.12.025
Gil-Castell O, Badia JD, Ontoria-Oviedo I, Castellano D, Sepúlveda P, Ribes-Greus A (2020) Polycaprolactone/gelatin-based scaffolds with tailored performance: in vitro and in vivo validation. Mater Sci Eng C Mater Biol Appl 107:110296. https://doi.org/10.1016/j.msec.2019.110296
Cui Z, Yang B, Li RK (2016) Application of biomaterials in cardiac repair and regeneration. Engineering 2:141–148
Vunjak-Novakovic G, Lui KO, Tandon N, Chien KR (2011) Bioengineering heart muscle: a paradigm for regenerative medicine. Annu Rev Biomed Eng 13:245–267
Pattar SS, Fatehi Hassanabad A, Fedak PWM (2019) Acellular extracellular matrix bioscaffolds for cardiac repair and regeneration. Front Cell Dev Biol 7:63. https://doi.org/10.3389/fcell.2019.00063
Zhang W, Du A, Liu S, Lv M, Chen S (2021) Research progress in decellularized extracellular matrix-derived hydrogels. Regen Ther 18:88–96. https://doi.org/10.1016/j.reth.2021.04.002
Tan B, Gan S, Wang X, Liu W, Li X (2021) Applications of 3D bioprinting in tissue engineering: advantages, deficiencies, improvements, and future perspectives. J Mater Chem B. https://doi.org/10.1039/d1tb00172h
Sung K, Patel NR, Ashammakhi N, Nguyen KL (2021) 3-Dimensional bioprinting of cardiovascular tissues: Emerging technology. JACC Basic Transl Sci 6:467–482. https://doi.org/10.1016/j.jacbts.2020.12.006
Acknowledgements
Dr. T. Hemalatha (No.R.12014/33/2020-HR) gratefully acknowledges Department of Health Research, Ministry of Health and Family Welfare, Government of India, for the Young Scientist fellowship. Dr. M. Aarthy thanks ICMR for the Research Associate fellowship, and Ms. P. Suryalakshmi thanks DST for the Senior Research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hemalatha, T., Aarthy, M., Pandurangan , . et al. A deep dive into the darning effects of biomaterials in infarct myocardium: current advances and future perspectives. Heart Fail Rev 27, 1443–1467 (2022). https://doi.org/10.1007/s10741-021-10144-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10144-3