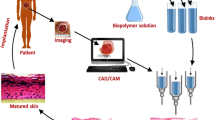
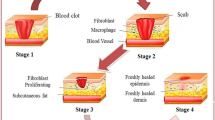
Abstract
The shortage of skin for grafting continues to be a major problem in the treatment of serious skin injuries. 3D bioprinting provides a new way to solve this problem. However, current 3D printed skin is less effective in treatment of large wounds because of severe shrinkage and scarring. In this study, bionically designed bilayer skin was fabricated using an extrusion-based bioprinter and a gelatin/sodium alginate/gelatin methacrylate hydrogel with excellent physical and biological properties. Full-thickness skin wounds were created in the back of nude mice and treated with bioprinted skin or hydrogel. Bioprinted skin accelerated wound healing, reduced wound contraction and scarring, and facilitated wound skin epithelialization compared with the bioprinted hydrogel or untreated wound. The skin from the wound was collected 28 days after grafting for histology and immunofluorescence analysis. The thickness of the dermis and epidermis of the bioprinted skin was similar to that of nude mice. Microvascular formation in the dermis and dense keratinocytes in the epidermis of the bioprinted skin were observed. This study provides a potential treatment strategy for reducing skin contraction and scar in large skin wounds.
Similar content being viewed by others
References
Jorgensen A M, Varkey M, Gorkun A, Clouse C, Xu L, Chou Z, Murphy S, Molnar J, Lee S J, Yoo J J, Soker S, Atala A. Bioprinted skin recapitulates normal collagen remodeling in full-thickness wounds. Tissue Engineering: Part A, 2020, 26, 512–526.
Krug E. A WHO Plan for Burn Prevention and Care. WHO Press, Geneva, Switzerland, 2008, 1–23.
Varkey M, Visscher D O, van Zuijlen P P M, Atala A, Yoo J J. Skin bioprinting: The future of burn wound reconstruction? Burns & Trauma, 2019, 7, 1–12.
Mohamed Haflah N H, Ng M H, Mohd Yunus M H, Naicker A S, Htwe O, Abdul Razak K A, Idrus R. Massive traumatic skin defect successfully treated with autologous, bilayered, tissue-engineered myderm skin substitute. Journal of Bone and Joint Surgery, 2018, 8, 1–4.
Nayak S, Dey S, Kundu S C. Skin equivalent tissue-engineered construct: Co-cultured fibroblasts/keratinocytes on 3D matrices of sericin hope cocoons. Plos One, 2013, 8, e74779.
Michael S, Sorg H, Peck C T, Koch L, Deiwick A, Chichkov B, Vogt P M, Reimers K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. Plos One, 2013, 8, e57741.
Zidaric T, Milojevic M, Gradisnik L, Stana Kleinschek K, Maver U, Maver T. Polysaccharide-based bioink formulation for 3D bioprinting of an in vitro model of the human dermis. Nanomaterials, 2020, 10, 2–18.
Wang R, Wang Y H, Yao B, Hu T, Li Z, Huang S, Fu X B. Beyond 2D: 3D bioprinting for skin regeneration. International Wound Journal, 2019, 16, 134–138.
Veen V C, Wal M B, Leeuwen M C, Ulrich M M, Middelkoop E. Biological background of dermal substitutes. Burns, 2010, 36, 305–321.
Nguyen D Q, Potokar T S, Price P. An objective long-term evaluation of integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns, 2010, 36, 23–28.
Ryssel H, Gazyakan E, Germann G, Ohlbauer M. The use of MatriDerm in early excision and simultaneous autologous skin grafting in burns-a pilot study. Burns, 2008, 34, 93–97.
Still J, Glat P, Silverstein P, Griswold J, Mozingo D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns, 2003, 29, 837–841.
Dantzer E, Braye F M. Reconstructive surgery using an artificial dermis (Integra): Results with 39 grafts. British Journalof Plastic Surgery, 2001, 54, 659–664.
Wang Y W, Beekman J, Hew J, Jackson S, Issler-Fisher A C, Parungao R, Lajevardi S S, Li Z, Maitz P K M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Advanced Drug Delivery Reviews, 2018, 123, 3–17.
Halim A S, Khoo T L, Mohd Yussof S J. Biologic and synthetic skin substitutes: An overview. Indian Journal of Plastic Surgery, 2010, 43, 23–28.
Enoch S, Shaaban H, Dunn K W. Informed consent should be obtained from patients to use products (skin substitutes) and dressings containing biological material. Journal of Medical Ethics, 2005, 31, 2–6.
Middelkoop E, Sheridan R L. Skin Substitutes and ‘the next level’. Total Burn Care, 2018, 15, 167–173.
Donnelly H, Salmeron-Sanchez M, Dalby M J. Designing stem cell niches for differentiation and self-renewal. Journal of The Royal Society Interface, 2018, 15, 1–18.
Daly A C, Prendergast M E, Hughes A J, Burdick J A. Bioprinting for the Biologist. Cell, 2021, 184, 18–32.
Singh M, Jonnalagadda S. Advances in bioprinting using additive manufacturing. European Journal of Pharmaceutical Sciences, 2020, 143, 105167.
Derr K, Zou J Y, Luo K, Song M J, Sittampalam G S, Zhou C, Michael S, Ferrer M, Derr P. Fully three-dimensional bioprinted skin equivalent constructs with validated morphology and barrier function. Tissue Engineering: Part C, 2019, 25, 334–343.
Liu X, Michael S, Bharti K, Ferrer M, Song M J. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication, 2020, 12, 035002.
Admane P, Gupta A C, Jois P, Roy S, Chandrasekharan Lakshmanan C, Kalsi G, Bandyopadhyay B, Ghosh S. Direct 3D bioprinted full-thickness skin constructs recapitulate regulatory signaling pathways and physiology of human skin. Bioprinting, 2019, 15, e00051.
Park J A, Lee H R, Park S Y, Jung S. Self-organization of fibroblast-laden 3D collagen microstructures from inkjet-printed cell patterns. Advanced Biosystems, 2020, 4, 1900280.
Zhang H B, Yong S, Tianlong X, Ruixue Y, Shimo Y, Wei J, Zhang W. Tyrosinase doped bioink for 3D bioprinting of living skin constructs. Biomedical Materials, 2018, 13, 035008.
Kim G, Ahn S, Kim Y, Cho Y, Chun W. Coaxial structured collagen-alginate scaffolds: fabrication, physical properties, and biomedical application for skin tissue regeneration. Journal of Materials Chemistry, 2011, 21, 6165–6172.
Baltazar T, Merola J, Catarino C M, Xie C B, Kirkiles-Smith N, Lee V, Hotta S Y K, Dai G, Xu X, Ferreira F C, Saltzman W M, Pober J S, Karande P. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Engineering: Part A, 2019, 201, 1–12.
Kim B S, Kwon Y W, Kong J S, Park G T, Gao G, Han W, Kim M B, Lee H, Kim J H, Cho D W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials, 2018, 168, 38–53.
Ng W L, Qi J T Z, Yeong W Y, Naing M W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication, 2018, 10, 025005.
Cubo N, Garcia M, del Canizo J F, Velasco D, Jorcano J L. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication, 2017, 9, 015006.
Yoon H, Lee J S, Yim H, Kim G, Chun W. Development of cell-laden 3D scaffolds for efficient engineered skin substitutes by collagen gelation. RSC Advances, 2016, 6, 21439–21447.
Kim B S, Gao G, Kim J Y, Cho D W. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Advanced Healthcare Materials, 2018, 8, 1801019.
Min D, Lee W, Bae I-H, Lee T R, Croce P, Yoo S-S. Bioprinting of biomimetic skin containing melanocytes. Experimental Dermatology, 2017, 27, 453–459.
Huang S, Yao B, Xie J F, Fu X B. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomaterialia, 2016, 32, 170–177.
Liu N B, Huang S, Yao B, Xie J F, Wu X, Fu X B. 3D bioprinting matrices with controlled pore structure and release function guide in vitro self-organization of sweat gland. Scientific Reports, 2016, 6, 34410.
Kim B S, Lee J S, Gao G, Cho D W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication, 2017, 9, 025034.
Cheng R Y, Eylert G, Gariepy J M, He S, Ahmad H, Gao Y, Priore S, Hakimi N, Jeschke M G, Gunther A. Handheld instrument for wound-conformal delivery of skin precursor sheets improves healing in full-thickness burns. Biofabrication, 2020, 12, 025002.
Albanna M, Binder K W, Murphy S V, Kim J, Qasem S A, Zhao W X, Tan J, El-Amin I B, Dice D D, Marco J, Green J, Xu T, Skardal A, Holmes J H, Jackson J D, Atala A, Yoo J J. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Scientific Reports, 2019, 9, 1856.
Hakimi N, Cheng R, Leng L, Sotoudehfar M, Ba P Q, Bakhtyar N, Amini-Nik S, Jeschke M G, Gunther A. Handheld skin printer: in situ formation of planar biomaterials and tissues. Lab on a Chip, 2018, 18, 1440–1451.
Stefanie Michael H S, Claas-Tido Peck, Lothar Koch, Andrea Deiwick, Boris Chichkov, Peter M. Vogt, Kerstin Reimers. Tissue engineered skin substitutes created by laserassisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. Plos One, 2013, 8, e57741.
Yanez M, Rincon J, Dones A, Carmelo De Maria, Gonzales R, Boland T. In vivo assessment of printed microvasculature in a bilayer skin graf to treat full-thickness wounds thickness wounds. Tissue Engineering: Part A, 2014, 21, 224–233.
Bayat A, McGrouther D A, Ferguson M W J. Skin scarring. British Medical Journal, 2003, 326, 88–92.
Gabbiani G, Ryan G B, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia, 1971, 27, 549–550.
Pharmd A D, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair and Regeneration, 2005, 13, 7–12.
Zuijlen P P M v, Vries H J C d, Lamme E N, Coppens J E, Marle J V, Kreis R W, Middelkoop E. Morphometry of dermal collagen orientation by Fourier analysis is superior to multi-observer assessment. The Journal of Pathology, 2002, 198, 284–291.
Grant C A, Twigg P C, Tobin D J. Static and dynamic nanomechanical properties of human skin tissue using atomic force microscopy: Effect of scarring in the upper dermis. Acta Biomaterialia, 2012, 8, 4123–4129.
Yannas I V, Tzeranis D S, So P T C. Regeneration of injured skin and peripheral nerves requires control of wound contraction, not scar formation. Wound Repair and Regeneration, 2017, 25, 177–191.
Yannas I V, Tzeranis D S, So P T C. Regeneration mechanism for skin and peripheral nerves clarified at the organ and molecular scales. Current Opinion in Biomedical Engineering, 2018, 6, 1–7.
Lian Q, Zhao T Z, Jiao T, Huyan Y G, Gu H, Gao L. Direct-writing process and in vivo evaluation of prevascularized composite constructs for muscle tissue engineering application. Journal of Bionic Engineering, 2020, 17, 457–468.
Yoon S, Park J A, Lee H R, Yoon W H, Hwang D S, Jung S. Inkjet-spray hybrid printing for 3D freeform fabrication of multilayered hydrogel structures. Advanced Healthcare Materials, 2018, 7, 1800050.
Zhou F F, Hong Y, Liang R J, Zhang X, Liao Y G, Jiang D M, Zhang J Y, Sheng Z X, Xie C, Peng Z, Zhuang X H, Bunpetch V, Zou Y W, Huang W W, Zhang Q, Alakpa E V, Zhang S F, Ouyang H W. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials, 2020, 258, 120287.
Soto Veliz D, Zhang H, Toivakka M. Stacking up: A new approach for cell culture studies. Biomaterials Science, 2019, 7, 3249–3257.
Sato K, Sato M, Yokoyama M, Hirai M, Furuta A. Influence of culture conditions on cell proliferation in a microfluidic channel. Analytical Sciences, 2019, 35, 49–56.
Puente P, Ludena D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Experimental Cell Research, 2014, 322, 1–11.
Lee K C, Dretzke J, Grover L, Logan A, Moiemen N. A systematic review of objective burn scar measurements. Burns & Trauma, 2016, 4, 1–33.
DeBruler D M, Zbinden J C, Baumann M E, Blackstone B N, Malara M M, Bailey J K, Supp D M, Powell H M. Early cessation of pressure garment therapy results in scar contraction and thickening. Plos One, 2018, 13, e0197558.
Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Research & Therapy, 2019, 10, 111.
Liu T, Qiu C, Ben C, Li H, Zhu S. One-step approach for full-thickness skin defect reconstruction in rats using minced split-thickness skin grafts with Pelnac overlay. Burns Trauma, 2019, 7, 19.
Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evidence-Based Complementary and Alternative Medicine, 2019, 2019, 2684108.
Rodriguez J, Boucher F, Lequeux C, Josset-Lamaugarny A, Rouyer O, Ardisson O, Rutschi H, Sigaudo-Roussel D, Damour O, Mojallal A. Intradermal injection of human adipose-derived stem cells accelerates skin wound healing in nude mice. Stem Cell Research Therapy, 2015, 6, 241.
Acknowledgment
This work was supported by the National Key R & D Program of China (No. 2018YFE0207900) and the Development projects of Key research of People’s Liberation Army (No. BWS17J036, 18-163-13-ZT-003-011-01) and the National Natural Science Foundation of China (51835010 and 51375371). The authors would like to thank Heng Gu, Feng Xu and Guorui Jin from Xi’an Jiaotong University and Dahai Hu from Air Force Medical University for their suggestions on animal experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lian, Q., Jiao, T., Zhao, T. et al. 3D Bioprinted Skin Substitutes for Accelerated Wound Healing and Reduced Scar. J Bionic Eng 18, 900–914 (2021). https://doi.org/10.1007/s42235-021-0053-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-021-0053-8