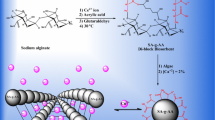
Abstract
Ammonia pollution of natural water can promote adverse undesirable environmental impacts on aquatic fauna, human and environment. Different techniques were elaborated for treatment of this pollution. Recently, polymer hydrogels have been successfully used as adsorbents of ammonium ions. Among the polysaccharides that can be used in the wastewater treatment, the alginates have the ability to adsorb various pollutants in the flexible channels by electrostatic interaction between the \({\text{NH}}_{4}^{ + }\) ions in aqueous solution and the –COO– groups of the polymeric network. Alginates are natural biopolymers; they could be obtained from the brown seaweeds, extraction of alginates is often effectuated in a sodium form related to their fast solubility in cold water. In this work, sodium alginate solution was crosslinked by calcium divalent cations in the beads forms. The resulting beads are roughly spherical, when they are swollen; they become bigger in size, they were characterized by several techniques, which revealed that the sample contains different mineral and organic functional groups. Indeed, optimization of the contact time, temperature, pH, initial adsorbate concentration and initial adsorbent concentration has been studied. So, the maximal removal efficiency was 47.72% after 60 min, the favorable temperature of adsorption was 30°C where the maximal removal efficiency was 69.54%, the optimal pH is 7 with a maximum removal efficiency of 69.54%. Moreover, the removal efficiency decreased considerably from 69.54 to 34.26% at initial EAC varied from 10 mg-\({\text{NH}}_{4}^{ + }\)/L to 1000 mg-\({\text{NH}}_{4}^{ + }\)/L and an optimal initial adsorbent amount of 0.1 g where the removal efficiency was 69.54% and the equivalence EAC was 30.46 mg-\({\text{NH}}_{4}^{ + }\)/L. Furthermore, study of adsorption isotherms indicated that the process was obeyed to both models of Langmuir and Freundlich with R2 values of 0.994 and 0.98 respectively, with a maximal adsorption capacity of 58.82 mg/g that calculated from Langmuir model. Finally, the spectral and the structural analyses after the adsorption process proved this phenomenon and allowed to apply these crosslinked beads-A in the treatment of waters charged with ammonium ions.




Similar content being viewed by others
REFERENCES
Li, X., Lin, C., Wang, Y., Zhao, M., and Hou, Y., Clinoptilolite adsorption capability of ammonia in pig farm, Procedia Environ. Sci., 2010, vol. 2, pp. 1598–1612.
Hasanoǧlu, A., Romero, J., Pérez, B., and Plaza, A., Ammonia removal from wastewater streams through membrane contactors: Experimental and theoretical analysis of operation parameters and configuration, Chem. Eng. J., 2010, vol. 160, pp. 530–537.
Alshameri, A., He, H., Zhu, J., Xi, Y., Zhu, R., Ma, L., and Tao, Q., Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms, Appl. Clay Sci., 2018, vol. 159, pp. 83–93.
Alshameri, A., Ibrahim, A., Assabri, A.M., Lei, X., Wang, H., and Yan, C., The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics, Powder Technol., 2014, vol. 258, pp. 20–31.
Cruz, H., Luckman, P., Seviour, T., Verstraete, W., Laycock, B., and Pikaar, I., Rapid removal of ammonium from domestic wastewater using polymer hydrogels, Sci. Rep., 2018, vol. 8. https://doi.org/10.1038/s41598-018-21204-4
Ma, Z., Li, Q., Yue, Q., Gao, B., Li, W., Xu, X., and Zhong, Q., Adsorption removal of ammonium and phosphate from water by fertilizer controlled release agent prepared from wheat straw, Chem. Eng. J., 2011, vol. 171, pp. 1209–1217.
Fertah, M., Belfkira, A., Dahmane, E., Taourirte, M., and Brouillette, F., Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed, Arab. J. Chem., 2017, vol. 10, no. 2, pp. 3707–3714.
Viswanathan, S. and Nallamuthu, T., Extraction of sodium alginate from selected seaweeds and their physiochemical and biochemical properties, Int. J. Innovative Sci., Eng. Technol., 2014, vol. 3, no. 4, pp. 10999–11003.
Aksas, H., Etude cinétique et thermodynamique de l’adsorption des métaux lourds par l’utilisation des adsorbants naturels, PhD Thesis, Boumerdes: Univ. of Boumerdes, 2013.
Amirouche, L., Etude du pouvoir de sorption du cuivre (II), du zinc (II) et des polyphénols par les bentonites sous l’effet des irradiations micro-ondes, MSc Thesis, Tizi Ouzou: Univ. of Tizi-Ouzou, 2011.
Rodier, J., Bazin, C., Broutin, J.P., Chambon, P., Champsaur, H., and Rodi, L., L’Analyse de l’Eau. Eaux Naturelles, Eaux Résiduaires, Eau de Mer: Chimie, Physico-Chimie, Microbiologie, Biologie, Interprétation des Résultats, 8th ed., Paris: Dunod, 2005, pp. 149–155.
Angar, Y., Djelali, N.E., and Kebbouche-Gana, S., Kinetic and thermodynamic studies of the ammonium ions adsorption onto natural Algerian bentonite, J. Desalin. Water Treat., 2016, vol. 57, pp. 25696–25704.
Langmuir, I.L., The constitution and fundamental properties of solids and liquids, J. Am. Chem. Soc., 1916, vol. 38, pp. 2221–2295.
Saltalı, K., Sarı, A., and Aydın, M., Removal of ammonium ion from aqueous solution by natural Turkish (Yıldızeli) zeolite for environmental quality, J. Hazard. Mater., 2007, vol. 141, pp. 258–263.
Angar, Y., Djelali, N.E., and Kebbouche-Gana, S., Investigation of ammonium adsorption on Algerian natural bentonite, Environ. Sci. Pollut. Res., 2017, vol. 24, pp. 11078–11089.
Daemi, H. and Barikani, M., Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles, Sci. Iran., 2012, vol. 19, no. 6, pp. 2023–2028.
Elmotasem, H., Chitosan-alginate blend films for the transdermal delivery of meloxicam, Asian J. Pharm. Sci., 2008, vol. 3, no. 1, pp. 12–29.
Gierszewska-Drużyńska, M. and Ostrowska-Czubenko, J., The effect of ionic crosslinking on thermal properties of hydrogel chitosan membranes, Prog. Chem. Appl. Chitin Its Deriv., 2010, vol. 15, pp. 25–31.
Ely, A., Synthése et propriétés de biosorbants á base d’argiles encapsulées dans des alginates: Application au traitement des eaux, PhD Thesis, Limoges: Univ. of Limoges, 2010.
Zheng, Y. and Wang, A., Evaluation of ammonium removal using a chitosan-g-poly (acrylic acid)/rectorite hydrogel composite, J. Hazard. Mater., 2009, vol. 171, pp. 671–677.
Zheng, Y., Zhang, J., and Wang, A., Fast removal of ammonium nitrogen from aqueous solution using chitosan-g-poly(acrylic acid)/attapulgite composite, Chem. Eng. J., 2009, vol. 155, pp. 215–222.
Zheng, Y., Liu, Y., and Wang, A., Fast removal of ammonium ion using a hydrogel optimized with response surface methodology, Chem. Eng. J., 2011, vol. 171, pp. 1201–1208.
Karadag, D., Koc, Y., Turan, M., and Armagan, B., Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite, J. Hazard. Mater., 2006, vol. 136, pp. 604–609.
Chen, J.P., Chua, M.L., and Zhang, B., Effects of competitive ions, humic acid, and pH on removal of ammonium and phosphorous from the synthetic industrial effluent by ion exchange resins, Waste Manage., 2002, vol. 22, pp. 711–719.
ACKNOWLEDGMENTS
The authors would like to thank also Dr. Ighil Ali M. for its relevant remarks and its careful reading of this manuscript.
Funding
This work was financed by University of M’Hamed Bougara of Boumerdes. We likewise greatly appreciate the help of SONATRACH for the provided analyses to this study. So, the constructive comments from Prof. Aliouch D., Dr. Khemili S., Dr. Belounes O. and Dr. Gana M.L. have helped us to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yassmina Angar, Djelali, NE. & Kebbouche-Gana, S. Crosslinked Calcium Alginates Porous Beads for Adsorption Removal of Ammonium Ions. J. Water Chem. Technol. 43, 218–227 (2021). https://doi.org/10.3103/S1063455X21030024
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X21030024