Abstract
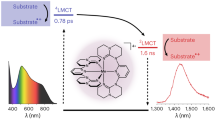
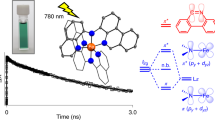
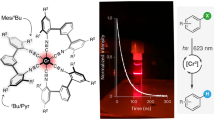
Precious metal complexes with the d6 valence electron configuration often exhibit luminescent metal-to-ligand charge transfer (MLCT) excited states, which form the basis for many applications in lighting, sensing, solar cells and synthetic photochemistry. Iron(ii) has received much attention as a possible Earth-abundant alternative, but to date no iron(ii) complex has been reported to show MLCT emission upon continuous-wave excitation. Manganese(i) has the same electron configuration as that of iron(ii), but until now has typically been overlooked in the search for cheap MLCT luminophores. Here we report that isocyanide chelate ligands give access to air-stable manganese(i) complexes that exhibit MLCT luminescence in solution at room temperature. These compounds were successfully used as photosensitizers for energy- and electron-transfer reactions and were shown to promote the photoisomerization of trans-stilbene. The observable electron transfer photoreactivity occurred from the emissive MLCT state, whereas the triplet energy transfer photoreactivity originated from a ligand-centred 3π–π* state.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All pertinent experimental procedures, materials, methods and characterization data (NMR spectroscopy, electrospray ionization mass spectrometry, X-ray diffraction, as well as optical spectroscopic and electrochemical data) are provided in this article and its Supplementary Information. Crystallographic data for [ZnCl2(Lbi)·0.5C2H4Cl2] have been deposited at the Cambridge Crystallographic Data Centre under deposition number CCDC 2047767. Copies of data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Costa, R. D. et al. Luminescent ionic transition-metal complexes for light-emitting electrochemical cells. Angew. Chem. Int. Ed. 51, 8178–8211 (2012).
Hagfeldt, A., Boschloo, G., Sun, L. C., Kloo, L. & Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 110, 6595–6663 (2010).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Magnuson, A. et al. Biomimetic and microbial approaches to solar fuel generation. Acc. Chem. Res. 42, 1899–1909 (2009).
Heinemann, F., Karges, J. & Gasser, G. Critical overview of the use of Ru(ii) polypyridyl complexes as photosensitizers in one-photon and two-photon photodynamic therapy. Acc. Chem. Res. 50, 2727–2736 (2017).
McCusker, J. K. Electronic structure in the transition metal block and its implications for light harvesting. Science 363, 484–488 (2019).
Liu, Y. Z., Persson, P., Sundström, V. & Wärnmark, K. Fe N-heterocyclic carbene complexes as promising photosensitizers. Acc. Chem. Res. 49, 1477–1485 (2016).
Zhang, W. K. et al. Tracking excited-state charge and spin dynamics in iron coordination complexes. Nature 509, 345–348 (2014).
Auböck, G. & Chergui, M. Sub-50-fs photoinduced spin crossover in Fe(bpy)32+. Nat. Chem. 7, 629–633 (2015).
Carey, M. C., Adelman, S. L. & McCusker, J. K. Insights into the excited state dynamics of Fe(ii) polypyridyl complexes from variable-temperature ultrafast spectroscopy. Chem. Sci. 10, 134–144 (2019).
Zhang, K., Ash, R., Girolami, G. S. & Vura-Weis, J. Tracking the metal-centered triplet in photoinduced spin crossover of Fe(phen)32+ with tabletop femtosecond M-edge X-ray absorption near-edge structure spectroscopy. J. Am. Chem. Soc. 141, 17180–17188 (2019).
Chábera, P. et al. Feii hexa N-heterocyclic carbene complex with a 528 ps metal-to-ligand charge-transfer excited-state lifetime. J. Phys. Chem. Lett. 9, 459–463 (2018).
Braun, J. D. et al. Iron(ii) coordination complexes with panchromatic absorption and nanosecond charge-transfer excited state lifetimes. Nat. Chem. 11, 1144–1150 (2019).
Duchanois, T. et al. NHC-based iron sensitizers for DSSCs. Inorganics 6, 63 (2018).
Förster, C. & Heinze, K. Photophysics and photochemistry with earth-abundant metals—fundamentals and concepts. Chem. Soc. Rev. 49, 1057–1070 (2020).
Chábera, P. et al. A low-spin Feiii complex with 100-ps ligand-to-metal charge transfer photoluminescence. Nature 543, 695–699 (2017).
Kjær, K. S. et al. Luminescence and reactivity of a charge-transfer excited iron complex with nanosecond lifetime. Science 363, 249–253 (2019).
Wood, C. J. et al. A comprehensive comparison of dye-sensitized NiO photocathodes for solar energy conversion. Phys. Chem. Chem. Phys. 18, 10727–10738 (2016).
Young, E. R. & Oldacre, A. Iron hits the mark. Science 363, 225–226 (2019).
Büldt, L. A., Guo, X., Vogel, R., Prescimone, A. & Wenger, O. S. A tris(diisocyanide)chromium(0) complex is a luminescent analog of Fe(2,2′-bipyridine)32+. J. Am. Chem. Soc. 139, 985–992 (2017).
Mann, K. R. et al. Electronic structures and spectra of hexakisphenylisocyanide complexes of Cr0, Mo0, W0, Mni, and Mnii. Inorg. Chim. Acta 16, 97–101 (1976).
Plummer, D. T. & Angelici, R. J. Synthesis and characterization of homoleptic complexes of the chelating bidentate isocyano ligand tert-BuDiNC. Inorg. Chem. 22, 4063–4070 (1983).
Treichel, P. M., Firsich, D. W. & Essenmacher, G. P. Manganese(i) and chromium(0) complexes of phenyl isocyanide. Inorg. Chem. 18, 2405–2409 (1979).
Hamze, R. et al. Eliminating nonradiative decay in Cui emitters: >99% quantum efficiency and microsecond lifetime. Science 363, 601–606 (2019).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, 450–461 (2019).
Di, D. W. et al. High-performance light-emitting diodes based on carbene–metal–amides. Science 356, 159–163 (2017).
Gernert, M. et al. Cyclic (amino)(aryl)carbenes enter the field of chromophore ligands: expanded π system leads to unusually deep red emitting Cui compounds. J. Am. Chem. Soc. 142, 8897–8909 (2020).
Liu, W. P. & Ackermann, L. Manganese-catalyzed C–H activation. ACS Catal. 6, 3743–3752 (2016).
Elangovan, S. et al. Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat. Commun. 7, 8 (2016).
Mukherjee, A. et al. Manganese-catalyzed environmentally benign dehydrogenative coupling of alcohols and amines to form aldimines and H2: a catalytic and mechanistic study. J. Am. Chem. Soc. 138, 4298–4301 (2016).
Sampson, M. D. et al. Manganese catalysts with bulky bipyridine ligands for the electrocatalytic reduction of carbon dioxide: eliminating dimerization and altering catalysis. J. Am. Chem. Soc. 136, 5460–5471 (2014).
Bourrez, M., Molton, F., Chardon-Noblat, S. & Deronzier, A. Mn(bipyridyl)(CO)3Br: an abundant metal carbonyl complex as efficient electrocatalyst for CO2 reduction. Angew. Chem. Int. Ed. 50, 9903–9906 (2011).
Henke, W. C., Otolski, C. J., Moore, W. N. G., Elles, C. G. & Blakemore, J. D. Ultrafast spectroscopy of [Mn(CO)3] complexes: tuning the kinetics of light-driven CO release and solvent binding. Inorg. Chem. 59, 2178–2187 (2020).
Zhang, Y. et al. Delayed fluorescence from a zirconium(iv) photosensitizer with ligand-to-metal charge-transfer excited states. Nat. Chem. 12, 345–352 (2020).
Pal, A. K., Li, C. F., Hanan, G. S. & Zysman-Colman, E. Blue-emissive cobalt(iii) complexes and their use in the photocatalytic trifluoromethylation of polycyclic aromatic hydrocarbons. Angew. Chem. Int. Ed. 57, 8027–8031 (2018).
Harris, J. P. et al. Near-infrared 2Eg → 4A2g and visible LMCT luminescence from a molecular bis-(tris(carbene)borate) manganese(IV) complex. Can. J. Chem. 95, 547–552 (2017).
Drance, M. J. et al. Terminal coordination of diatomic boron monofluoride to iron. Science 363, 1203–1205 (2019).
Sattler, W., Henling, L. M., Winkler, J. R. & Gray, H. B. Bespoke photoreductants: tungsten arylisocyanides. J. Am. Chem. Soc. 137, 1198–1205 (2015).
Mann, K. R., Gray, H. B. & Hammond, G. S. Excited-state reactivity patterns of hexakisarylisocyano complexes of chromium(0), molybdenum(0), and tungsten(0). J. Am. Chem. Soc. 99, 306–307 (1977).
Herr, P., Glaser, F., Büldt, L. A., Larsen, C. B. & Wenger, O. S. Long-lived, strongly emissive, and highly reducing excited states in Mo(0) complexes with chelating isocyanides. J. Am. Chem. Soc. 141, 14394–14402 (2019).
Harris, R. K. & Mann, B. E. NMR and the Periodic Table (Academic, 1978).
Brown, A. M., McCusker, C. E. & McCusker, J. K. Spectroelectrochemical identification of charge-transfer excited states in transition metal-based polypyridyl complexes. Dalton Trans. 43, 17635–17646 (2014).
Gawelda, W. et al. Ultrafast nonadiabatic dynamics of Feii(bpy)32+ in solution. J. Am. Chem. Soc. 129, 8199–8206 (2007).
Murtaza, Z. et al. Energy-transfer in the inverted region—calculation of relative rate constants by emission spectral fitting. J. Phys. Chem. 98, 10504–10513 (1994).
Baba, A. I., Shaw, J. R., Simon, J. A., Thummel, R. P. & Schmehl, R. H. The photophysical behavior of d6 complexes having nearly isoenergetic MLCT and ligand localized excited states. Coord. Chem. Rev. 171, 43–59 (1998).
Taffarel, E., Chirayil, S., Kim, W. Y., Thummel, R. P. & Schmehl, R. H. Coexistence of ligand localized and MLCT excited states in a 2-(2′-pyridyl)benzo[g]quinoline complex of ruthenium(ii). Inorg. Chem. 35, 2127–2131 (1996).
Anne, A., Hapiot, P., Moiroux, J., Neta, P. & Savéant, J. M. Oxidation of 10-methylacridan, a synthetic analog of NADH, and deprotonation of its cation radical—convergent application of laser flash-photolysis and direct and redox catalyzed electrochemistry to the kinetics of deprotonation of the cation radical. J. Phys. Chem. 95, 2370–2377 (1991).
Singh-Rachford, T. N. & Castellano, F. N. Photon upconversion based on sensitized triplet–triplet annihilation. Coord. Chem. Rev. 254, 2560–2573 (2010).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Kvapilova, H. et al. Electronic excited states of tungsten(0) arylisocyanides. Inorg. Chem. 54, 8518–8528 (2015).
Arias-Rotondo, D. M. & McCusker, J. K. The photophysics of photoredox catalysis: a roadmap for catalyst design. Chem. Soc. Rev. 45, 5803–5820 (2016).
Dorn, M. et al. A vanadium(iii) complex with blue and NIR-II spin–flip luminescence in solution. J. Am. Chem. Soc. 142, 7947–7955 (2020).
Acknowledgements
O.S.W. thanks the Swiss National Science Foundation (grant nos 200021_178760 and 206021_157687) for financial support. C.K. acknowledges a Novartis University of Basel Excellence Scholarship for Life Sciences.
Author information
Authors and Affiliations
Contributions
P.H. carried out the synthetic, spectroscopic and electrochemical work and analysed data, C.K. provided guidance in the spectroscopic work, designed the photochemical studies and helped in data analysis, C.B.L. provided guidance in the synthetic, spectroscopic and electrochemical work, D.H. performed the NMR studies and O.S.W. conceived the project and provided guidance. All the authors contributed to the writing and editing of the manuscript and gave approval to its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
NMR spectroscopy, electrospray ionization mass spectrometry, X-ray diffraction, as well as optical spectroscopic and electrochemical data; Supplementary Schemes 1 and 2, Figs. 1–56 and references 1–23.
Supplementary Data
Crystallographic data for [ZnCl2(Lbi)·0.5C2H4Cl2], CCDC 2047767.
Source data
Source Data Fig. 2
UV-vis absorption and luminescence spectra.
Source Data Fig. 3
UV-vis transient absorption, time-resolved emission, cyclic voltammetry and spectro-electrochemistry data.
Source Data Fig. 4
UV-vis absorption, luminescence and excitation spectra.
Source Data Fig. 5
UV-vis transient absorption and time-resolved luminescence data.
Rights and permissions
About this article
Cite this article
Herr, P., Kerzig, C., Larsen, C.B. et al. Manganese(i) complexes with metal-to-ligand charge transfer luminescence and photoreactivity. Nat. Chem. 13, 956–962 (2021). https://doi.org/10.1038/s41557-021-00744-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00744-9
This article is cited by
-
Janus-type emission from a cyclometalated iron(iii) complex
Nature Chemistry (2023)
-
Photoredox-active Cr(0) luminophores featuring photophysical properties competitive with Ru(II) and Os(II) complexes
Nature Chemistry (2023)
-
Photoinduced manganese-catalysed hydrofluorocarbofunctionalization of alkenes
Nature Synthesis (2022)
-
Luminescent Metal Complexes for Bioassays in the Near-Infrared (NIR) Region
Topics in Current Chemistry (2022)
-
Luminescent Metal Complexes as Emerging Tools for Lipid Imaging
Topics in Current Chemistry (2022)