Abstract
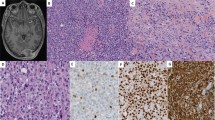
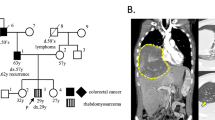
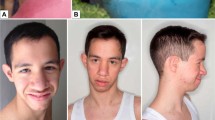
PAX3/7-FOXO1 fusion-negative alveolar rhabdomyosarcoma (ARMS) developed in a patient presenting with intellectual disability and dysmorphic facial features. Whole exome sequencing analysis of a germline sample identified a PACS1 c.607 C>T de novo variant and the patient was diagnosed with Schuurs-Hoeijmakers syndrome (SHS). SHS is a rare disease characterized by intellectual disability and dysmorphic facial features, among various physical abnormalities, due to PACS1 c.607 C>T de novo variant. Due to the rarity of the SHS, diagnosis based on phenotypic information is difficult. To date, there have been no previous reports describing malignancy associated with SHS. Comprehensive somatic mutation analysis revealed a unique pattern of genetic alterations in the PAX3/7-FOXO1 fusion-negative ARMS tumor, including mutations in the oncogene, HRAS; MYOD1, a molecule essential for muscle differentiation; and KMT2C and TET1, genes encoding factors involved in epigenetic regulation. Although the role of PACS1 in tumorigenesis is unclear, it is reported to function in apoptosis regulation. Our case suggests that PACS1 could have a novel role in oncogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Narod SA, Hawkins MM, Robertson CM, Stiller CA. Congenital anomalies and childhood cancer in Great Britain. Am J Hum Genet. 1997;60:474–85.
Agha MM, Williams JI, Marrett L, To T, Zipursky A, Dodds L. Congenital abnormalities and childhood cancer. Cancer. 2005;103:1939–48.
Norwood MS, Lupo PJ, Chow EJ, Scheurer ME, Plon SE, Danysh HE, et al. Childhood cancer risk in those with chromosomal and non-chromosomal congenital anomalies in Washington State: 1984–2013. PLoS ONE. 2017;12:e0179006.
Bjorge T, Cnattingius S, Lie RT, Tretli S, Engeland A. Cancer risk in children with birth defects and in their families: a population based cohort study of 5.2 million children from Norway and Sweden. Cancer Epidemiol Biomark Prev. 2008;17:500–6.
Altmann AE, Halliday JL, Giles GG. Associations between congenital malformations and childhood cancer. A register-based case-control study. Br J Cancer. 1998;78:1244–9.
Williamson D, Missiaglia E, de Reynies A, Pierron G, Thuille B, Palenzuela G, et al. Fusion gene-negative alveolar rhabdomyosarcoma is clinically and molecularly indistinguishable from embryonal rhabdomyosarcoma. J Clin Oncol. 2010;28:2151–8.
Adachi T, Kawamura K, Furusawa Y, Nishizaki Y, Imanishi N, Umehara S, et al. Japan’s initiative on rare and undiagnosed diseases (IRUD): towards an end to the diagnostic odyssey. Eur J Hum Genet. 2017;25:1025–8.
Schuurs-Hoeijmakers JH, Oh EC, Vissers LE, Swinkels ME, Gilissen C, Willemsen MA, et al. Recurrent de novo mutations in PACS1 cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. Am J Hum Genet. 2012;91:1122–7.
Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, et al. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–16.
Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–6.
Agaram NP, LaQuaglia MP, Alaggio R, Zhang L, Fujisawa Y, Ladanyi M, et al. MYOD1-mutant spindle cell and sclerosing rhabdomyosarcoma: an aggressive subtype irrespective of age. A reappraisal for molecular classification and risk stratification. Mod Pathol. 2019;32:27–36.
Kohsaka S, Shukla N, Ameur N, Ito T, Ng CK, Wang L, et al. A recurrent neomorphic mutation in MYOD1 defines a clinically aggressive subset of embryonal rhabdomyosarcoma associated with PI3K-AKT pathway mutations. Nat Genet. 2014;46:595–600.
Seki M, Nishimura R, Yoshida K, Shimamura T, Shiraishi Y, Sato Y, et al. Integrated genetic and epigenetic analysis defines novel molecular subgroups in rhabdomyosarcoma. Nat Commun. 2015;6:7557.
Cheng L, Pandya PH, Liu E, Chandra P, Wang L, Murray ME, et al. Integration of genomic copy number variations and chemotherapy-response biomarkers in pediatric sarcoma. BMC Med Genom. 2019;12:23.
Shern JF, Chen L, Chmielecki J, Wei JS, Patidar R, Rosenberg M, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4:216–31.
Brasacchio D, Alsop AE, Noori T, Lufti M, Iyer S, Simpson KJ, et al. Epigenetic control of mitochondrial cell death through PACS1-mediated regulation of BAX/BAK oligomerization. Cell Death Differ. 2017;24:961–70.
Mani C, Tripathi K, Luan S, Clark DW, Andrews JF, Vindigni A, et al. The multifunctional protein PACS-1 is required for HDAC2- and HDAC3-dependent chromatin maturation and genomic stability. Oncogene. 2020;39:2583–96.
Seto MT, Bertoli-Avella AM, Cheung KW, Chan KY, Yeung KS, Fung JL, et al. Prenatal and postnatal diagnosis of Schuurs-Hoeijmakers syndrome: case series and review of the literature. Am J Med Genet A. 2021;185:384–9.
Acknowledgements
We thank the affected individuals and their families for participating in this study. This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas from MEXT (3905-A02) and Japan agency for medical research and development (AMED) (grant number 19ck0106467).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ohkawa, T., Nishimura, A., Kosaki, K. et al. PAX3/7-FOXO1 fusion-negative alveolar rhabdomyosarcoma in Schuurs-Hoeijmakers syndrome. J Hum Genet 67, 51–54 (2022). https://doi.org/10.1038/s10038-021-00965-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-021-00965-3