Abstract
Background
There is increasing appreciation of the association of obesity beyond co-morbidities, such as cancers, Type 2 diabetes, hypertension, and stroke to also impact upon the muscle to give rise to sarcopenic obesity. Phenotypic knowledge of obesity is crucial for profiling and management of obesity, as different fat—subcutaneous adipose tissue depots (SAT) and visceral adipose tissue depots (VAT) have various degrees of influence on metabolic syndrome and morbidities. Manual segmentation is time consuming and laborious. Study focuses on the development of a deep learning-based, complete data processing pipeline for MRI-based fat analysis, for large cohort studies which include (1) data augmentation and preprocessing (2) model zoo (3) visualization dashboard, and (4) correction tool, for automated quantification of fat compartments SAT and VAT.
Methods
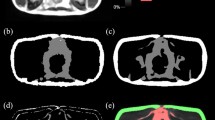
Our sample comprised 190 healthy community-dwelling older adults from the Geri-LABS study with mean age of 67.85 ± 7.90 years, BMI 23.75 ± 3.65 kg/m2, 132 (69.5%) female, and mainly Chinese ethnicity. 3D-modified Dixon T1-weighted gradient-echo MR images were acquired. Residual global aggregation-based 3D U-Net (RGA-U-Net) and standard 3D U-Net were trained to segment SAT, VAT, superficial and deep subcutaneous adipose tissue depots (SSAT and DSAT). Manual segmentation from 26 subjects was used as ground truth during training. Data augmentations, random bias, noise and ghosting were carried out to increase the number of training datasets to 130. Segmentation accuracy was evaluated using Dice and Hausdorff metrics.
Results
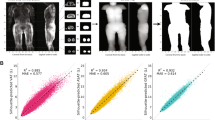
The accuracy of segmentation was SSAT:0.92, DSAT:0.88 and VAT:0.9. Average Hausdorff distance was less than 5 mm. Automated segmentation significantly correlated R2 > 0.99 (p < 0.001) with ground truth for all 3-fat compartments. Predicted volumes were within ± 1.96SD from Bland–Altman analysis.
Conclusions
DL-based, comprehensive SSAT, DSAT, and VAT analysis tool showed high accuracy and reproducibility and provided a comprehensive fat compartment composition analysis and visualization in less than 10 s.









Similar content being viewed by others
Availability of data and material
Data cannot be shared due to National policy.
Code availability
Can be made available based on the approval from the funding institute.
Change history
04 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10334-021-00956-7
References
World Health Organization (2016) World Health Organization. http://www.who.int/mediacentre/factsheets/fs311/en/
Murray CJL (2021) Institute for health metrics and evaluation. http://www.healthdata.org/news-release/nearly-one-third-world%E2%80%99s-population-obese-or-overweight-new-data-show
Djalalinia S, Qorbani M, Peykari N, Kelishadi R (2015) Health impacts of obesity. Pakistan J Med Sci 31:239–242
U.S National Library—The World’s Largest Medical Library (2016) https://www.ncbi.nlm.nih.gov/pubmedhealth/behindtheheadlines/news/2016-07-14-obesity-now-a-leading-cause-of-death-especially-in-men/. Accessed 14 Jul 2016
Khor EQE, Lim JP, Tay L, Yeo A, Yew S, Ding YY, Lim WS (2020) Obesity definitions in sarcopenic obesity: Differences in prevalence, agreement and association with muscle function. J Frailty Aging 9:37–43
Article on SAMS. http://medicine.nus.edu.sg/medi/doc/education/combating_diabetes.pdf
Khoo CM, Leow MK, Sadananthan SA, Lim R, Venkataraman K, Khoo EY, Velan SS, Ong YT, Kambadur R, McFarlane C, Gluckman PD, Lee YS, Chong YS, Tai ES (2014) Body fat partitioning does not explain the interethnic variation in insulin sensitivity among Asian ethnicity: the Singapore adults metabolism study. Diabetes 63(3):1093–1102. https://doi.org/10.2337/db13-1483
Sadananthan SA, Tint MT, Michael N, Aris IM, Loy SL, Lee KJ, Shek LP, Yap FKP, Tan KH, Godfrey KM, Leow MK, Lee YS, Kramer MS, Gluckman PD, Chong YS, Karnani N, Henry CJ, Fortier MV, Velan SS (2019) Association between early life weight gain and abdominal fat partitioning at 4.5 years is sex, ethnicity, and age dependent. Obesity (Silver Spring) 27(3):470–478. https://doi.org/10.1002/oby.22408
NCD-RisC (2014) Risk factor collaboration. http://www.ncdrisc.org/obesity-prevalence-map.html
Positano V, Gastaldelli A, Sironi A, Santarelli M, Lombardi M, Landini L (2004) An accurate and robust method for unsupervised assessment of abdominal fat by MRI. J Magn Reson Imaging 20(4):684–689
Sussman D, Yao J, Summers R (2010) Automated measurement and segmentation of abdominal adipose tissue in MRI. In: IEEE international symposium on biomedical imaging: from nano to macro, pp 936–939
Kullberg J, Ahlström H, Johansson L, Frimmel H (2007) Automated and reproducible segmentation of visceral and subcutaneous adipose tissue from abdominal MRI. Int J Obes 31:1806–1817
Joshi AA, Hu H, Leahy R, Goran M, Nayak K (2013) Automatic intra-subject registration-based segmentation of abdominal fat from water-fat MRI. J Magn Reson Imaging 37(2):423–430
Leinhard OD, Johansson A, Rydell J, Smedby Ö, Nyström F, Lundberg P, Borga M (2008) Quantitative abdominal fat estimation using MRI. In: 2008 19th international conference on pattern recognition, pp 1–4
Mosbech TH, Pilgaard K, Vaag A, Larsen R (2011) Automatic segmentation of abdominal adipose tissue in MRI. In: Heyden A, Kahl F (eds) Image analysis. SCIA 2011. Lecture notes in computer science, vol 6688. Springer, Berlin
Sadananthan SA, Prakash B, Leow MK, Khoo C, Chou H, Venkataraman K, Khoo E, Lee Y, Gluckman P, Tai E, Velan S (2015) Automated segmentation of visceral and subcutaneous (deep and superficial) adipose tissues in normal and overweight men. J Magn Reson Imaging 41(4):924–934
Ronneberger O, Fischer P, Brox T (2015) U-Net: convolutional networks for biomedical image segmentation. arXiv:1505.04597
Estrada S, Lu R, Conjeti S, Orozco-Ruiz X, Panos-Willuhn J, Breteler MM, Reuter M (2020) FatSegNet: a fully automated deep learning pipeline for adipose tissue segmentation on abdominal dixon MRI. Magn Reson Med 83(4):1471–1483
Chew J, Tay L, Lim JP, Yeo A, Yew S, Tan CN, Ding YY, Lim WS (2019) Serum myostatin and IGF-1 as gender-specific biomarkers of frailty and low muscle mass in community-dwelling older adults. J Nutr Health Aging 23(10):979–986
Pérez-García F, Sparks R, Ourselin S (2020) TorchIO: a Python library for efficient loading, preprocessing, augmentation, and patch-based sampling of medical images in deep learning. arXiv:2003.04696
Uçar MK, Nour M, Sindi H, Polat K (2020) The effect of training and testing process on machine learning in biomedical datasets. Math Prob Eng 2020:2836236
Le Ba T, Khanh D-P, Ho N-H, Yang H-J, Baek E-T, Lee G, Kim S-H, Yoo SB (2020) Enhancing U-Net with spatial-channel attention gate for abnormal tissue segmentation in medical imaging. Appl Sci 10(17):5729
Shelhamer E, Long J, Darrell T (2017) Fully convolutional networks for semantic segmentation. IEEE Trans Pattern Anal Mach Intell 39(4):640–651
Badrinarayanan V, Kendall A, Cipolla R (2017) SegNet: a deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans Pattern Anal Mach Intell 39:2481–2495
Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O (2016) 3D U-Net: learning dense volumetric segmentation from sparse annotation. arXiv:1606.06650
Zeiler MD, Krishnan D, Taylor GW, Fergus R (2010) Deconvolutional networks. IEEE CVPR 2010(2528–2535):5539957
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521:436–444
Isensee F, Jaeger PF, Kohl SAA et al (2021) nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods 18:203–211. https://doi.org/10.1038/s41592-020-01008-z
He F, Liu T, Tao D (2020) Why ResNet works? Residuals generalize. IEEE Trans Neural Netw Learn Syst 31:5349–5362
Ba J, Kiros JR, Hinton GE (2016) Layer normalization. arXiv:1607.06450
Agarap AF (2018) Deep learning using rectified linear units (ReLU). arXiv:1803.08375
Zhou Z, Siddiquee MM, Tajbakhsh N, Liang J (2018) U-Net++: a nested u-net architecture for medical image segmentation. DLMIA/ML-CDS@MICCAI, arXiv:1807.10165
Srivastava N, Hinton GE, Krizhevsky A, Sutskever I, Salakhutdinov R (2014) Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res 15:1929–1958
Wan R, Zhu Z, Zhang X, Sun J (2020) Spherical motion dynamics of deep neural networks with batch normalization and weight decay. arXiv:2006.08419
Kingma DP, Ba J (2015) Adam: a method for stochastic optimization. arXiv:1412.6980
Boer PD, Kroese DP, Mannor S, Rubinstein RY (2005) A tutorial on the cross-entropy method. Ann Oper Res 134:19–67. https://doi.org/10.1007/s10479-005-5724-z
Braiek HB, Khomh F (2019) TFCheck: a tensorflow library for detecting training issues in neural network programs. In: 2019 IEEE 19th international conference on software quality, reliability and security (QRS), pp 426–433
Sadananthan SA, Prakash B, Leow MK, Khoo CM, Chou H, Venkataraman K, Khoo E, Lee YS, Gluckman P, Tai ES, Velan SS (2015) Automated segmentation of visceral and subcutaneous (deep and superficial) adipose tissues in normal and overweight men. J Magn Reson Imaging JMRI 41(4):924–934
Zhao Z, Kuang X, Zhu Y, Liang Y, Xuan Y (2020) Combined kernel for fast GPU computation of Zernike moments. J Real Time Image Proc 1–14
Shamir RR, Duchin Y, Kim J, Sapiro G, Harel NY (2019) Continuous dice coefficient: a method for evaluating probabilistic segmentations. BioRxiv
Demerath E, Shen W, Lee M, Choh A, Czerwinski S, Siervogel R, Towne B (2007) Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr 85(2):362–368
Shen W, Punyanitya M, Chen J, Gallagher D, Albu J, Pi-Sunyer X, Lewis C, Grunfeld C, Heymsfield S, Heshka S (2007) Visceral adipose tissue: relationships between single-slice areas at different locations and obesity-related health risks. Int J Obesity 31(5):763–769
Ng A, Wai D, Tai E, Ng K, Chan L (2012) Visceral adipose tissue, but not waist circumference is a better measure of metabolic risk in Singaporean Chinese and Indian men. Nutr Diabetes 2(8):e38
Thomas EL, Saeed N, Hajnal J, Brynes A, Goldstone A, Frost G, Bell JD (1998) Magnetic resonance imaging of total body fat. J Appl Physiol 85(5):1778–1785
Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, Armamento-Villareal R, Qualls C (2017) Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med 376(20):1943–1955. https://doi.org/10.1056/NEJMoa1616338.s
Acknowledgements
Our sincere thanks to Singapore Bioimaging Consortium, A*STAR, and Tan Tock Seng Hospital for providing funds and data for conducting this study. We would also like to thank the support received from TechSource Systems Pte Ltd, especially Application Engineer Kevin Chng Jun Yan for his technical guidance and help rendered when needed during the development of the correction tool.
Author information
Authors and Affiliations
Contributions
KNB, CS worked on deep learning-based framework development, data processing, concept development, correction tool, and manuscript. LY developed a correction tool and assisted in the manuscript. CHT, WSL, and WC collected data (image acquisition), generated ground truth, and worked on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Ethics approval
Institutional Review Boards have approved the study and written consent was taken from the subjects.
Consent for publication
Authors consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhanu, P.K., Arvind, C.S., Yeow, L.Y. et al. CAFT: a deep learning-based comprehensive abdominal fat analysis tool for large cohort studies. Magn Reson Mater Phy 35, 205–220 (2022). https://doi.org/10.1007/s10334-021-00946-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-021-00946-9