Abstract
Background
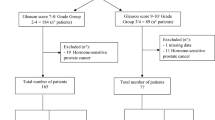
In a Phase 2 clinical trial, we aimed to determine the lutetium-177 [177Lu]-PSMA-617 activity and the clinical utility of levels of plasma androgen receptor (AR) gene in patients with heavily pretreated metastatic castration-resistant prostate cancer (mCRPC).
Methods
We determined AR copy number in pretreatment plasma samples. We used logistic regression to estimate the odds ratio (OR) and 95% confidence intervals (95% CIs) in order to evaluate the independent relevance of AR status and to evaluate patients with early progressive disease (PD) defined as treatment interruption occurring within 4 months after the start of 177Lu-PSMA-617.
Results
Twelve of the 15 (80%) with AR gene gain and 5 of the 25 (20%) patients with no gain of AR had early PD (p = 0.0002). The OR for patients without PSA response having AR gain was 3.69 (95% CI 0.83–16.36, p = 0.085). The OR for patients with early PD having AR gain was 16.00, (95% CI 3.23–79.27, p = 0.0007). Overall, median PFS and OS were 7.5 and 12.4 months, respectively. AR-gained had a significant shorter OS compared to AR-normal patients (7.4 vs 19.1 months, p = 0.020). No treatment interruptions due to adverse effects were reported.
Discussion
Plasma AR status helped to indicate mCRPC with early resistance to 177Lu-PSMA-617.
Trial registration
NCT03454750.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary information data.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Attard G, Parker C, Eeles RA, Schroder F, Tomlins SA, Tannock I. Prostate cancer. Lancet. 2016;387:70–82.
Quigley DA, Dang HX, Zhao SG, Lloyd P, Aggarwal R, Alumkal JJ, et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell. 2018;174:758–69.
Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22:369–78.
Mateo J, Seed G, Bertan C, Rescigno P, Dolling D, Figueiredo I, et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J Clin Invest. 2020;130:1743–51.
Salvi S, Casadio V, Conteduca V, Burgio SL, Menna C, Bianchi E, et al. Circulating cell-free AR and CYP17A1 copy number variations may associate with outcome of metastatic castration-resistant prostate cancer patients treated with abiraterone. Br J Cancer. 2015;112:1717–24.
Romanel A, Gasi Tandefelt D, Conteduca V, Jayaram A, Casiraghi N, Wetterskog D, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re10.
Conteduca V, Wetterskog D, Sharabiani MTA, Grande E, Fernandez-Perez MP, Jayaram A, et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: a multi-institution correlative biomarker study. Ann Oncol. 2017;28:1508–16.
Conteduca V, Jayaram A, Romero-Laorden N, Wetterskog D, Salvi S, Gurioli G, et al. Plasma androgen receptor and docetaxel for metastatic castration-resistant prostate cancer. Eur Urol. 2019;75:368–73.
Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-Labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–76.
Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–61.
Bravaccini S, Puccetti M, Bocchini M, Ravaioli S, Celli M, Scarpi E, et al. PSMA expression: a potential ally for the pathologist in prostate cancer diagnosis. Sci Rep. 2018;8:4254.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90.
Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44:1448–54.
Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. J Nucl Med. 2016;57:1170–6.
Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med. 2016;57:97S–104S.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3:1254–71.
Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3:1245–53.
Yin Y, Li R, Xu K, Ding S, Li J, Baek G, et al. Androgen receptor variants mediate DNA repair after prostate cancer irradiation. Cancer Res. 2017;77:4745–54.
Paganelli G, Sarnelli A, Severi S, Sansovini M, Belli ML, Monti M, et al. Dosimetry and safety of 177Lu PSMA-617 along with polyglutamate parotid glands protector: preliminary results in metastatic castration-resistant prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020;47:3008–17.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
Bakht MK, Lovnicki JM, Tubman J, Stringer KF, Chiaramonte J, Reynolds MR, et al. Differential expression of glucose transporters and hexokinases in prostate cancer with a neuroendocrine gene signature: a mechanistic perspective for FDG imaging of PSMA-suppressed tumors. J Nucl Med. 2020;61:904–10.
von Eyben FE, Singh A, Zhang J, Nipsch K, Meyrick D, Lenzo N, et al. 177Lu-PSMA radioligand therapy of predominant lymph node metastatic prostate cancer. Oncotarget. 2019;10:2451–61.
Castro E, Romero-Laorden N, Del Pozo A, Lozano R, Medina A, Puente J, et al. PROREPAIR-B: a prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37:490–503.
Conteduca V, Oromendia C, Vlachostergios PJ, Hackett A, Thomas C, Caseet A, et al. Clinical and molecular analysis of patients treated with prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy. J Clin Oncol. 2019;37:272.
Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76:469–78.
de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wülfing C, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506518.
Hofman MS, Emmett L, Sandhu SK, Iravani A, Joshua AM, Goh JC, et al. TheraP: a randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: Initial results (ANZUP protocol 1603). J Clin Oncol. 2020;38:5500.
Conteduca V, Castro E, Wetterskog D, Scarpi E, Jayaram A, Romero-Laorden N, et al. Plasma AR status and cabazitaxel in heavily treated metastatic castration-resistant prostate cancer. Eur J Cancer. 2019;116:158–68.
De Vincentis G, Gerritsen W, Gschwend JE, Hacker M, Lewington V, O’Sullivan JM, et al. Advances in targeted alpha therapy for prostate cancer. Ann Oncol. 2019;30:1728–39.
Funding
This work was partially supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) and Ricerca Finalizzata Italian Ministry of Health (no grant number applicable).
Author information
Authors and Affiliations
Contributions
Conception and design: UDG, GP. Development of methodology: UDG, MS, SS, MM, FF, DC, GP. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): UDG, MS, SS, SN, MM, GG, FF, CC, VC, MC, VDI, DC, FM, FEvE, GA, GP. Analysis and interpretation of data (e.g. statistical analysis, biostatistics, computational analysis): SN, MM, GG, FF, CC, VDI. Writing, review, and/or revision of the manuscript: UDG, MS, SS, SN, MM, GG, FF, CC, VC, MC, VDI, DC, FM, FEvE, GA, GP. Administrative, technical, or material support (i.e. reporting or organising data, constructing databases): MM, FF. Study supervision: UDG, MS, SS, GP.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from each patient prior to entry into the study. The study was conducted in compliance with the principles of the Declaration of Helsinki and local ethical and legal requirements. The protocol and informed consent were approved by the Institutional Review Board of Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST), Meldola, Italy (NCT03454750).
Competing interests
UDG reports honoraria, consulting fees, or travel support from Merck, Bristol Myers Squibb, Janssen, Pfizer, Novartis, Astellas, Bayer, Sanofi, and Novartis and grant support from Merck and Amgen. VC reports honoraria, consulting fees, or travel support Bayer, Astellas, Janssen-Cilag, and Sanofi-Aventis. GA certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: GA reports receiving commercial research grants from Janssen, Arno Therapeutics, and Innocrin Pharma; has received honoraria and/or travel support from the speakers’ bureaus of Janssen, Astellas, Sanofi-Aventis, and Roche/Ventana; has received travel support from Pfizer, Abbott Laboratories, Bayer Healthcare, and Essa Pharmaceuticals; has ownership interest (including patents) in The Institute of Cancer Research Rewards to Inventors; and is a consultant for/advisory board member of Janssen-Cilag, Veridex, Bayer Healthcare, Roche/Ventana, Astellas, Medivation, Pfizer, Novartis, Millennium Pharma, Abbott Laboratories, and Essa Pharma.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Giorgi, U., Sansovini, M., Severi, S. et al. Circulating androgen receptor gene amplification and resistance to 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: results of a Phase 2 trial. Br J Cancer 125, 1226–1232 (2021). https://doi.org/10.1038/s41416-021-01508-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01508-5
This article is cited by
-
Radiotheranostics in oncology: current challenges and emerging opportunities
Nature Reviews Clinical Oncology (2022)
-
Lutetium Lu 177 Vipivotide Tetraxetan: First Approval
Molecular Diagnosis & Therapy (2022)