Abstract
The adsorption of methanol in HZSM-5 at low temperatures has long been regarded as an associative process involving hydrogen bonding to the acidic zeolite hydroxyl groups. Recent studies employing inelastic neutron scattering spectroscopy (INS) have reported that complete dissociation to methoxylate the zeolite occurs at 298 K, and infrared evidence for a partial dissociation at 298 K has also been described. Here we investigate the apparent contradictions between different techniques, using a combination of INS, infrared spectroscopy and solid-state NMR spectroscopy, including isotopic substitution experiments. Different possible explanations are proposed and considered; we conclude that at room temperature methanol is very largely associatively adsorbed, although the presence of some small extent (>1%) of methoxylation cannot be ruled out.
Similar content being viewed by others
1 Introduction
The interaction between methanol and acidic zeolite catalysts is important because of its relevance to Methanol-to-Hydrocarbons (MTH) technology. In MTH, methanol derived from coal or natural gas is converted into more valuable industrially relevant hydrocarbons, including light olefins and methylated aromatics. HZSM-5 zeolite was one of the first catalysts used for this reaction [1, 2]. The mechanisms of hydrocarbon formation from methanol have been widely studied [3,4,5,6,7], and it is generally accepted that the first step is the reaction of methanol with the zeolite’s Brønsted sites to generate methoxy species, as shown in Scheme 1 [8,9,10].
For example, Forester and Howe [10] carried out an in-situ FTIR study of methanol in HZSM-5. At room temperature, the spectra showed only bands characteristic of hydrogen bonded species. Pulsing methanol under flowing helium at temperatures above 373 K showed methoxylation of the acid sites, revealed by loss of the zeolite ν(OH) band and appearance of new bands in the CH stretching and bending regions. The room temperature interaction of methanol with HZSM-5 was subsequently studied in more detail by Zecchina et al. [11]. The infrared spectra varied with the methanol loading. At low loadings (< 1 molecule per acid site) hydrogen bonding of individual methanol molecules to zeolite OH groups was observed, manifested in the appearance of an ABC triplet due to interception of the broad OH stretching mode of the hydrogen bonded zeolite OH groups by Fermi resonance with overtones of δ(OH) and γ(OH) modes [11,12,13]. At higher coverages, formation of protonated clusters of methanol through complete proton transfer from the OH groups was reported.
In contrast, a more recent study of methanol interaction on both ZSM-5 and HY using inelastic neutron scattering (INS) spectroscopy and quasielastic neutron scattering (QENS) reported complete methanol dissociation to methoxy species at room temperature on HZSM-5 [14]. Methanol was dosed on the dehydrated zeolite at room temperature under He flow. The QENS spectra of the dosed ZSM-5 showed no broadening of the elastic peak suggesting no movement in the zeolite framework, which was contrasted with a dosed HY sample where significant broadening was observed [14]. The INS spectra of the dosed ZSM-5 showed no deformation or stretching modes of zeolite or methanol OH groups, whereas in HY both stretching and deformation modes of OH groups were detected, consistent with hydrogen bonding of methanol. Similar QENS evidence for lack of mobility at room temperature on the QENS time scale was later reported for methanol in both a fresh HZSM-5 catalyst and one used in an MTH reactor for three days at 623 K [15]. Very recently a more detailed QENS study has looked at the behaviour of methanol in HZSM-5 as a function of temperature and the aluminium content of the zeolite [16]. According to this study the fraction of immobile methanol depends on both temperature and the number of acid sites in the zeolite.
A DRIFTS study by Matam et al. has suggested that it is possible to see formation of methoxy groups from methanol in HZSM-5 at room temperature by infrared spectroscopy [17], although the methoxy bands are difficult to distinguish due to the intense ABC structure of the hydrogen bonded methanol. The same group recently showed by a combination of DRIFTS and computational modelling that room temperature methoxylation is methanol loading dependent, finding that methoxylation at room temperature does not occur at loadings of less than one mole of methanol per acid site [18].
In the present study we have attempted to rationalise the apparently contradictory results obtained from the different forms of spectroscopy by applying a combination of techniques (INS, DRIFTS and solid state NMR (ssNMR)) to look at methanol in the same HZSM-5 zeolite.
2 Experimental
The HZSM-5 zeolite (Si:Al ~ 30) was provided in powder form by Johnson Matthey and its characterisation can be found elsewhere [19]. Prior to use, the zeolite was calcined at 773 K for 12 h under static air. Prior to methanol adsorption the catalyst was dried at 623 K under flowing He (100 ml min−1). The INS experiments used the TOSCA [20] and MAPS [21] spectrometers that are located at the ISIS Pulsed Neutron and Muon Source (Oxfordshire, UK). The instruments are complementary: TOSCA provides good resolution spectra over the 0–2000 cm−1 range, while MAPS provides access to the C–H and O–H stretching regions (2000–4000 cm−1). The MAPS spectra were collected using the A-chopper package with a fixed incident energy of 650 meV (600 Hz) and 250 meV (400 Hz) [21]. The DRIFTS experiments used an Agilent Carey 680 FTIR spectrometer with a liquid nitrogen cooled MCT detector and a Harrick Praying Mantis DRIFTS environmental chamber accessory. 64 scans per spectrum were collected at a resolution of 4 cm−1. The ss-NMR experiments were carried out using two spectrometers: a Bruker Avance III (400 MHz) Fourier transform NMR spectrometer and a Bruker Avance Neo (600 MHz) NMR spectrometer. The 13C-NMR experiment used the HPDEC pulse sequence which does not rely on cross-polarisation and is therefore quantitative. The INS spectra were recorded below 20 K, the infrared and ss-NMR spectra were recorded at room temperature. Table 1 shows a summary of all the samples that were prepared.
2.1 Flow Dosed Methanol Experiments
The dried ZSM-5 was transferred to a flat aluminium gas handling cell in an argon filled glovebox, to prevent exposure to moisture. For flow dosed samples, the methanol dosing used a Dreschel bottle maintained at room temperature and with 1.1 l min−1 He flow as the carrier gas. For the flow dosed (FD) samples both CH3OH (Sigma Aldrich, 99.8% anhydrous) and CD3OH (Sigma Aldrich, 99.7 atom% D) were used as well as 13CH3OH (Sigma Aldrich, 99 atom% 13C). All of these samples were analysed by INS. The FD-13CH3OH was also analysed by DRIFTS and ss-NMR using the 600 MHz spectrometer. The FD-13CH3OH was dosed with a mixture of 14 wt% 13C-methanol in 12C-methanol. For the DRIFTS experiment, the Harrick cell dome reactor was charged with the post-reaction catalyst sample inside an argon filled glove box, before being transferred to the spectrometer. For the ss-NMR experiments, the ss-NMR rotor was packed inside the glove box and sealed in an airtight container before being transferred to the spectrometer. The aluminium flat gas handling cans used for the flow-dosed samples are compatible with use inside the INS spectrometer.
2.2 Exchanged Methanol Experiments
The dried zeolite was loaded into an aluminium gas handling cell in an argon filled glovebox as above. The CH3OD (Sigma Aldrich, 99.5 atom% D) was loaded on the zeolite in the same way as the flow dosed samples to a level of 15.3 molCH3OD/molZSM5, as determined gravimetrically. The sample was analysed using the MAPS spectrometer. The sample was then dosed with CH3OH (Sigma Aldrich, 99.8% anhydrous) in order to exchange the OD with the OH until saturation was reached, achieving a new loading of 15.7 molMeOH/molZSM5, and returned to the MAPS spectrometer for collection of a post-exchange spectrum.
2.3 Static Dosed Methanol Experiments
1 ml of methanol was injected onto ~ 10 g of dried ZSM-5. The zeolite was then sealed in a desiccator with a methanol-saturated atmosphere at room temperature for a period of 70 h. The sample was then transferred to an Al flat can and the INS spectrum collected with both TOSCA and MAPS spectrometers. For the ss-NMR static dosed experiments, ZSM-5 was dried under vacuum and dosed with 13C enriched methanol via syringe injection. Two different loadings were chosen: (i) 6.6 μl methanol (16.5 molMeOH/molZSM-5), which is comparable to the loading concentration used for the INS experiments and (ii) 1.2 μl (3.3 molMeOH/molZSM-5), which is comparable to conditions used in infrared experiments by Forester and Howe [10] 13C-NMR and 1H-NMR spectra were recorded for both samples. The 6.6 μl dosed sample was left sealed for an extra 1.5 h before collecting another set of 13C-NMR and 1H-NMR spectra in order to observe any changes with time, then heated to 423 K in order to observe partial methoxylation.
2.4 In-Situ Experiments
DRIFTS was used for flow dosed in-situ experiments of methanol loaded ZSM-5. The ZSM-5 was dried in-situ, using liquid nitrogen boil-off as the inert gas flow in order to minimise introduced moisture and hence avoid re-hydrating the zeolite. Methanol was then introduced into the system via manual injections through a septum into the inlet gas stream at 298 K and scans were completed both before and after the injections were made. The ZSM-5 was heated gradually at 5 K min−1 to 473 K in order to induce methoxylation before being cooled back down to room temperature. A second set of injections were completed at room temperature on the same sample.
3 Results and Discussion
This paper is structured as follows: in the first section we show the INS, DRIFTS and ss-NMR results that show apparent contradictions between the techniques. In subsequent sections we investigate possible scenarios that may reconcile the contradictory results. We conclude with a summary that describes how we believe this paradox can be resolved. To ensure consistency, the same ZSM-5 zeolite was used throughout the investigation. This particular ZSM-5 catalyst has been previously used for the MTH reaction with methanol [19], dimethyl ether [22] and methyl acetate [23] as feedstocks. As seen in the Electronic Supplementary Information (ESI) at 623 K with methanol it behaves as a conventional ZSM-5 catalyst with the products consisting of a range of methylated hydrocarbons, Figures S1–S3. However, when the catalyst was exposed to methanol at room temperature, other than the expected uptake of methanol, no reaction products were obtained and, importantly, no water was produced at any stage during the time-on-stream.
3.1 Evidence for and Against Methoxylation
3.1.1 Inelastic Neutron Experiment
The quantity of sample required for INS experiments limits the ways in which room temperature dosing can be completed. It must be noted that the quantity of the sample depends on the INS spectrometer used. However, even for the most sensitive instrument available (VISION at SNS); the sample size cannot match the quantity required for NMR or DRIFTS experiments (10–60 mg). Flow dosing the methanol via a Dreschel bottle is the simplest way to load the methanol onto the dried zeolite in an INS-compatible flow-through cell (12.15 molMeOH/molZSM5, ~ 4 MeOH/acid site). Figure 1 shows a comparison of solid methanol reference spectra obtained by both TOSCA and MAPS, as well as the spectra of the methanol dosed on ZSM-5 with the ZSM-5 contribution subtracted (FD-MEOH). The spectra before subtraction can be found in the ESI Figures S4–S6).
The characteristic OH-related modes of methanol are the stretch above 3200 cm−1 and the OH deformations at 700 cm−1 and 780 cm−1 (there is more than one mode because there are four molecules in the primitive cell of methanol [24]). In contrast, the FD-MEOH appears to have lost the OH functionality; neither the OH stretch or deformations are present. We note that the spectra are in complete agreement with those previously reported by O’Malley et al. in an INS and QENS study of methanol in a different ZSM-5 zeolite [14]. The apparent loss of the OH functionality is indeed consistent with room temperature methoxylation.
3.1.2 Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS)
DRIFTS spectra were collected for the dried ZSM-5, after the injection of methanol (6 injections of 2 μl methanol over 20 min) at room temperature and during flushing with inert gas for 60 min. Figure 2a shows the spectra of the blank ZSM-5, after methanol was injected and then flushed for 60 min under a high flow rate. The spectrum recorded on initial injection of methanol closely resembles that assigned by Zecchina et al. to a mixture of protonated methanol clusters and single methanol molecules hydrogen bonded to zeolite OH groups [11]. The spectra are dominated by the ABC triplet of hydrogen bonded methanol and a broad continuum beginning around 3300 cm−1. The difference spectra (i.e. the dried, bare zeolite used as the background spectrum) are shown in Fig. 2b–d. As seen in Fig. 2b, methanol interacts with all three types of zeolite OH groups (silanol groups, extra-framework AlOH, and Brønsted acid sites).
a Spectra of the blank ZSM-5 (black), ZSM-5 with methanol injected under flow (blue), the sample was then flushed under 100 ml min−1 of N2 (grey). A final spectrum was collected after 20 min of flushing (green). The spectra are unsubtracted, but the intensity has been normalised using the overtone band (1866 cm−1) on the blank ZSM-5. The ABC triplet is highlighted (A: green, B: purple, C: pink). The difference spectra of (a) are found in the ESI in Figure S7. Detailed view of: b the difference spectra in the acid site region (4000–3400 cm−1) c the difference spectra in the CH stretch region (3100–2600 cm−1) and d the difference spectra of the C–O stretch region where the PQR bands of gass phase methanol are found. A comparison of the difference spectrum with the spectra of liquid and gas phase methanol is shown in Figure S8.
On flushing at room temperature the broad OH band at ~ 3300 cm−1 is removed and there is an obvious upwards shift in the highest frequency ν(CH) band (Fig. 2c); the spectrum after flushing for 60 min closely resembles that assigned by Zecchina et al. [11] to single methanol molecules hydrogen bonded to zeolite OH groups i.e. the changes on flushing are those expected for reducing the amount of adsorbed methanol.
Difference spectra in the low frequency (1400 to 700 cm−1) region are difficult to interpret because of strong absorption by the zeolite lattice, perturbation of lattice modes by adsorption [25] and, in the specific case of diffuse reflectance spectra, distortions caused by dispersion effects. The very weak features appearing in the difference spectra in Fig. 2d are mostly artefacts. In the initial spectrum (blue) methanol vapour is still present in the cell as seen from the PQR vibrational bands of the methanol CO stretching mode at 1000–1100 cm−1, but these disappear on flushing, as expected. Matam et al. also report loss of methanol vapour bands on flushing but in this study we did not see the new band at 1004 cm−1 assigned by Matam et al. to the CO stretching mode of surface methoxy groups [17, 18]. In the CH stretching region surface methoxy groups in ZSM-5 have asymmetric and symmetric stretching modes at 2980 and 2868 cm−1 respectively [10], but these bands could not be distinguished in the spectra measured here from those of hydrogen bonded methanol.
3.1.3 Solid State NMR
The ss-NMR experiments were carried out via static dosing of 13C-methanol onto dried ZSM-5. Two loadings were used: the first (1.2 μl methanol) is comparable to that of previous studies in the literature [26] of about 3.3 molMEOH/molZSM-5; the second (6.6 μl methanol) is comparable to the INS dosing experiments, 16.5 molMEOH/molZSM-5. As seen in Fig. 3, the spectra for the room temperature loaded samples (black lines) at both methanol loadings showed a single Lorentzian line at 51.2 ppm, with no contribution from any other species present. This is attributed to hydrogen-bonded methanol [7, 26]. Figure 3a shows the NMR spectrum of the INS-comparable loaded ZSM-5 at room temperature and after heating to 423 K. After heating the ss-NMR spectra changes significantly.
ssNMR spectra a methanol dosing comparable to INS, and then heated at 423 K b methanol dosing comparable to Ref [9], and then evacuated to 0.3 mbar
Two signals are present: the major signal at 60.2 ppm is assigned to methoxy groups and/or dimethylether [7, 26], and the smaller signal at 51.2 ppm indicates some residual hydrogen bonded methanol in the zeolite. Figure 3b shows the methanol loaded ZSM5 at room temperature as well as the spectra of the sample after it has been evacuated to 0.3 mbar. Evacuating the sample did not cause a significant change in the spectra suggesting that the methanol is strongly adsorbed on the zeolite surface. Again, no signal was observed at 60 ppm which suggests that no methoxylation of the zeolite has occurred. Using an HPDEC pulse sequence, 13C ss-NMR is quantitative, so Fig. 3 enables determination of an upper limit for the amount of methoxylation that could take place and be unseen by ss-NMR. This is determined by the signal-to-noise ratio, which for the signal at 51.2 ppm in Fig. 3a is at least 100. This means that no more than 1% of the methyl groups could be present as methoxy groups in the sample before heating. We note that these results are in agreement with previous work [7, 26].
In summary, all of the spectra presented here (INS, ss-NMR and DRIFTS) are compatible with previous work in the literature. However, they present different aspects: (i) INS appears to indicate complete methoxylation with no hydrogen-bonded methanol present; (ii) ss-NMR shows only hydrogen-bonded methanol at room temperature, methoxylation only occurs on heating; while (iii) DRIFTS shows hydrogen-bonded methanol with, at most, traces of methoxy groups. In the following section we consider four scenarios that could reconcile these apparently disparate observations.
3.2 Possible Reconciliation of the Observed Differences Between Techniques
3.2.1 Scenario 1: The OH Modes in the INS Spectra are Hidden by the C-H Stretch and Bending Modes
The INS spectra seen in Fig. 1 show no OH-related peaks in either the deformation or the OH stretch region. However, the CH stretch peak is quite broad suggesting that the OH peak could be contributing to the total intensity. Hydrogen bonding not only broadens peaks but also shifts them. Strong hydrogen bonding shifts the deformation modes to higher wavenumber and the stretching modes to lower wavenumber [27]. It is conceivable that the OH deformation modes of methanol have upshifted under the CH3 rocking modes at 1100–1200 cm−1 and that the OH stretch modes have downshifted under the CH stretching modes. To investigate this possibility a deuterated methanol (CD3OH) was used to load the zeolite under exactly the same conditions as used for the spectra in Fig. 1 and the INS spectra measured. Figure 4 shows the solid CD3OH reference data obtained by both TOSCA and MAPS, as well as the spectra of the CD3OH dosed on ZSM-5, with the zeolite contribution subtracted (FD-CD3OH, 17.8 molCD3OH/molZSM-5, ~ 6 CD3OH/acid site).
From the TOSCA spectra spectra (Fig. 4a), it is seen that for the adsorbed molecule the OH deformation modes at 702 and 768 cm−1 are no longer present, whereas the CD3 rock at 895 cm−1 is still observed. There is a very broad peak (~ 800 cm−1) underlying the methyl deformations, which could be the broadened OH deformation peaks. The peaks at 1405 and 1455 cm−1 in the reference spectrum are assigned to OH bending modes; it is unclear if the broad feature observed at 1390 cm−1 for the adsorbed molecule is due to the OH bending modes broadened. From the MAPS reference spectrum of CD3OH (Fig. 4b), three prominent peaks are seen: one at 2220 cm−1 that is assigned to the unresolved C–D stretch modes, one at 2800 cm−1 assigned to the overtone of the OH in-plane bending modes found at 1405 cm−1 and, finally, the 3260 cm−1 feature assigned as the OH stretching mode [28].
From the MAPS spectrum of FD-CD3OH (Fig. 4b), the C-D stretch modes are weak but distinguishable, whereas the two OH assigned modes are either absent or broadened to > 1000 cm−1 width. This CD3OH experiment is thus inconclusive, and does not give a clear answer as to the interaction of methanol with the zeolite at room temperature.
3.2.2 Scenario 2: Both Hydrogen Bonded Species and Methoxy Species are Present
It is difficult to distinguish the two species via infrared spectroscopy due to the different extinction coefficients of ν(O–H) and ν(C–H). The DRIFTS experiments of Matam et al., have suggested that at saturation levels of methanol, methoxylation is possible [17, 18]. However, the spectra are dominated by the ABC triplet of hydrogen-bonded methanol. This potentially hides any methoxy peaks, as was seen in Fig. 3. To check this possibility, methanol was injected at room temperature, the zeolite heated to 523 K where methoxylation is expected to occur [8,9,10], cooled back to room temperature and the same amount of methanol injected again. Both the heating and injections occurred under a nitrogen flow of 10 ml min−1, with the total methanol injection each time being 12 μl. The spectra recorded are shown in Fig. 5.
a DRIFTS difference spectra, 6 injections of 2 μl methanol at room temperature at 20 ml/min nitrogen flow, heated to 523 K at 5 K min−1, cooled to room temperature and 6 further injections, 2 μl methanol. Difference spectra obtained by subtracting the spectrum of the dehydrated zeolite. b Detailed view of the CH region (3300–2500 cm−1) c Detailed view of the 650–1500 cm−1 region
The first room temperature spectrum is that of hydrogen bonded methanol, with the ABC triplet clearly dominating the spectra. The extinction coefficient of the OH stretching modes is clearly much larger than that of the CH stretching modes. A crude estimate of the total integrated area of the CH stretching modes compared with that of the OH stretching modes (ESI Figure S9) indicates a difference of at least 20-fold.
With progressive heating, the bands due to hydrogen-bonded methanol diminish. In the CH stretching region (Fig. 5b) the bands observed match those reported in the literature for hydrogen bonded dimethylether [11, 26], at 3010, 2971, 2945 and 2840 cm−1. The two bands expected for surface methoxy groups (asymmetric and symmetric stretching modes at 2980 and 2868 cm−1 respectively are not clearly seen, although their presence can be inferred from the formation of dimethylether. The low frequency difference spectra show the appearance of a new band at 870 cm−1 on heating to 523 K. We suggest this band may be the symmetric C–O–C stretch of adsorbed dimethylether, down shifted from the gas-phase value of 928 cm−1 by hydrogen bonding [29]. On cooling to room temperature and reinjection of methanol, the spectrum obtained is almost indistinguishable from that obtained initially, showing loss of the 870 cm−1 band of dimethylether and appearance again of hydrogen bonded methanol. Any methoxy groups which may have been formed at 523 K are completely obscured by the hydrogen bonded methanol.
3.2.3 Scenario 3: The Difference Between INS and NMR is Due to the Dosing Differences
Under the right conditions, both NMR and INS are quantitative techniques, which makes their discrepancies in this issue the more surprising. One difference between the techniques is the sample mass required: NMR having an approximate sample size of 0.5 g and INS usually needing up to 12 g of sample to obtain good S/N spectra with the TOSCA and MAPS instruments. Due to this disparity it could be argued that a sample size effect (during the dosing of the samples) is responsible for methoxylation occurring in the INS sample. Another possible explanation is that the INS samples have been prepared under flow conditions, whereas most NMR experiments have been performed with static dosing. Both of these possibilities are explored below.
Dosing effect The dosing effect was checked by preparing a static dosed ZSM-5 in large enough quantities for an INS measurement. In order to do that, the sample was dried and then kept in a desiccator containing a saturated vapour pressure of methanol for 70 h before being transferred into the spectrometer. Figure 6, shows the MAPS spectrum (SM-MEOH, 15.5 molMeOH/molZSM5, approximately 5 methanol molecules per acid site) recorded with an incident energy of 5244 cm−1. The stretching region does not show any differences from previous INS experiments. In the deformation region, there is a broad weak feature where the OH deformation peaks should be. Figure 6b shows the TOSCA spectrum of the same sample, which suggests that the broadness observed, is not due to the low resolution resulting from the use of 5243 cm−1 incident energy to observe the C–H and O–H stretch region. It is very broad, suggesting that the hydrogen bonding is very strong, but its intensity still does not match that expected for 15 mol of methanol per ZSM-5 unit cell.
It is also noteworthy that no water is observed in the INS spectrum at Fig. 6. Any water formed by methoxylation (Scheme 1) should remain in the zeolite in a static experiment and be observable. Water has very characteristic librational modes which occur at 400–800 cm−1 [30, 31] as well as characteristic HOH deformation and stretching modes which do not appear to be present, although distinguishing these from the OH modes of hydrogen bonded methanol may be problematical. The broadness observed in the deformation region could possibly be due to water present at low concentrations, although the high (for water) transition energy of 600–800 cm−1 strongly militates against this assignment. It is not possible from this experiment to know if it is due to a small quantity of methanol converting to methoxy or due to the dosing procedure. We note that there are no other peaks present that could be assigned to water (translational modes, H–O–H bend and stretch).
We therefore designed a different experiment in which a flow-dosed sample was produced from a 14 wt% 13C methanol in 12C-methanol mixture in sufficient quantity to be analysed firstly by INS and then with 13C NMR and DRIFTS. The total methanol loading is 17.8 molesMeOH/molesZSM-5 (approximately 6 methanols per acid site). The spectra obtained from this sample with the three different techniques are presented in Fig. 7. This experiment eliminates the possibility of different dosing conditions giving different results. The ss-NMR spectrum shows that there are no methoxy groups or dimethylether present in the 13C-methanol loaded zeolite sample, as there are no signals at 60 ppm. The DRIFTS spectrum is dominated by the bands due to hydrogen bonded methanol, as discussed above, and no bands due to methoxy groups can be distinguished. The INS spectrum shows similar features to those described above, with the possibility at least of weak broad shoulders in the OH deformation and OH stretching regions which could indicate the presence of hydroxyl groups.
3.2.4 Scenario 4: OH Peaks Present at INS Spectra are Broadened to the Point of No Detection
This hypothesis assumes that the hydrogen bonding is so strong that it causes the OH peaks to broaden out to the point that they become part of the baseline and are undetectable by INS spectroscopy. Hydrogen and deuterium have different neutron cross- sections and this allows us to test this possibility. A ZSM-5 sample was first loaded with CH3OD and the spectrum measured with the MAPS spectrometer. The same zeolite sample was then exposed to CH3OH to the same concentration and re-measured. If methoxylation had occurred, then there should be no difference between the spectra of the CH3OD and CH3OH exposed samples because the methoxy group has no deuterium incorporation. If methanol is present as a hydrogen-bonded species but with extremely broad OH-related bands, then the exchange of CH3OD for CH3OH should result in a raised baseline because of the larger total cross section of 1H (82.03 barn) versus 2H (7.64 barn) [32]. Figure 8 shows the results of this experiment. (Extended range spectra are shown in Figure S10). The sample was exposed to CH3OD first to a loading of 15.3 molCH3OD/molZSM-5, and then the same sample was exposed to a flow saturated with normal methanol (CH3OH). The sample mass increased to a loading of 15.7 molCH3OH/molZSM-5.
MAPS spectra of CH3OD dosed ZSM-5 vs exchanged CH3OH dosed ZSM-5. Both spectra were collected using the A-chopper package at an incident energy of a 2016.75 cm−1 b 5243.55 cm−1. c Detailed view at the 1000–1600 cm−1 of the pure CH3OD and CH3OH spectra collected via the TOSCA spectrometer for comparison purposes
It can be seen that the spectra do not overlap and that the CH3OH dosed spectra have gained intensity. This demonstrates that the methanol did not undergo methoxylation and that the methanol is present as a hydrogen-bonded species, albeit with extremely broad OH-related modes. This interpretation is also supported by the transition energies of the CH3 rocking modes [28]. Figure 8c compares the spectra of the pure CH3OD and CH3OH in the rocking mode region and it can be seen that the peaks occur at slightly different transition energies for the two isotopomers. For CH3OH they occur at 1120 and 1157 cm−1, whereas, for CH3OD they occur at 1167 and 1232 cm−1. Crucially, comparison with the spectra in Fig. 8a shows that this is also true of the CH3OD and CH3OH zeolite loaded spectra. This can only be the case if the CH3OD and CH3OH are intact in the zeolite.
4 Conclusions
This paper has shown that observing the OH functionality of methanol when methanol is loaded on ZSM-5 is complex with INS spectroscopy. When methanol is loaded on ZSM-5 at room temperature, both infrared and ss-NMR spectroscopies definitively show that the methanol OH functionality is still present and dominates both sets of spectra. With INS spectroscopy, the OH functionality is not immediately evident, which led to the previous conclusion that methanol is dissociated at room temperature. A small degree of methoxylation was suggested by infrared spectroscopy [17, 18] that could be attributed to differences in the experimental conditions employed. Theory suggests that methanol dissociation may be facilitated at higher loading and higher acid site density [18, 33]. However, our combined INS, DRIFTS and NMR studies on the same zeolite sample (Fig. 7) show at room temperature this occurs to a very limited extent. A variety of scenarios have been considered that could reconcile the apparent contradictions between the techniques. The explanation that best fits the observations is that methanol is largely (≥ 99%) present as the hydrogen-bonded species at ambient temperature. This is consistent with the DRIFTS (Fig. 5) and ss-NMR (Fig. 3) data. This is also consistent with the CD3OH experiment (Fig. 4), which was previously thought to be inconclusive due to the lack of an OH peak present within the spectrum. Concerning the INS spectra (Fig. 1), the OH-related modes have broadened to such an extent that they merge into the baseline so they are no longer clearly visible. This model also accounts for why there is no evidence of water in any of the INS spectra (Figs. 1, 4, 6, 7). It was originally assumed that if methoxylation had occurred, then the water generated would be flushed out of the zeolite. While this is conceivable for the flow dosed system, the water by-product should have still been present in the static dosed samples, which it was not (Fig. 6). It is possible that the water stretch and bend modes could be masked by methoxy modes, however, the very strong librational modes would occur at 400–800 cm−1, which is a region that is clear of interference.
The presence of very broad OH-related modes has some precedent in a study of KH2AsO4 [34] which is strongly hydrogen-bonded in the solid state. In this case, the O–H stretch mode extends over a range of ~ 2000 cm−1, although both the in-plane and out-of-plane As–O–H bending modes give distinct modes. For the adsorption system under consideration here, all of the methanol OH-related modes are broadened to at least 1000 cm−1, which makes them very difficult to identify. The authors believe this to be a previously unrecognised phenomenon for this adsorption system, which warrants further study.
References
Chang CD, Silvestri AJ (1977) The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. J Catal 47:249–259
Chang CD (1983) Hydrocarbons from methanol. Catal Rev 25:1–118
Haw JF, Song W, Marcus DM et al (2003) The mechanism of methanol to hydrocarbon catalysis. Acc Chem Res 36:317–326
Olsbye U, Svelle S, Lillerud KP et al (2015) The formation and degradation of active species during methanol conversion over protonated zeotype catalysts. Chem Soc Rev 44:7155–7176
Schulz H (2018) “Coking” of zeolites during methanol conversion: basic reactions of the MTO-, MTP- and MTG processes. Catal Lett 148:1263–1280
Yarulina I, Chowdhury A, Meirer F et al (2018) Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat Catal 1:398–411
Wang C, Xu J, Deng F (2020) Mechanism of methanol-to-hydrocarbon reaction over zeolites: a solid-state NMR perspective. ChemCatChem 12:965–980
Ono Y, Mori T (1981) Mechanism of methanol conversion into hydrocarbons over ZSM-5 zeolite. J Chem Soc Faraday Trans I 77:2209–2221
Wang W, Hunger M (2008) Reactivity of surface alkoxy species on acidic zeolite catalysts. Acc Chem Res 41:895–904
Forester TR, Howe RF (1987) In situ FTIR studies of methanol and dimethyl ether in ZSM-5. J Am Chem Soc 109:5076–5082
Zecchina A, Bordiga S, Spoto G et al (1996) IR spectroscopy of neutral and ionic hydrogen-bonded complexes formed upon interaction of CH3OH, C2H5OH, (CH3)2O, (C2H5)2O and C4H8O with H-Y, H-ZSM-5 and H-mordenite: comparison with analogous adducts formed on the H-Nafion superacidic membrane. J Chem Soc Faraday Trans 92:463–4875
Claydon MF, Sheppard N (1969) The nature of “A, B, C”-type infrared spectra of strongly hydrogen-bonded systems; Pseudo-maxima in vibrational spectra. J Chem Soc D Chem Comm 23:1431–1433
Palmenschikov AG, van Santen RA, Jänchen J et al (1993) CD3CN as a probe of Lewis and Brønsted acidity of zeolites. J Phys Chem 97:11071–11074
O’Malley AJ, Parker SF, Chutia A et al (2016) Room temperature methoxylation in zeolites: insight into a key step of the methanol to hydrocarbons process. Chem Commun 52:2897–2900
Matam SK, O’Malley AJ, Catlow CRA et al (2018) The effects of MTG catalysis on methanol mobility in ZSM-5. Catal Sci Technol 8:3304–3312
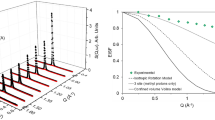
Omojola T, Silverwood IP, O’Malley AJ (2020) Molecular behaviour of methanol and dimethyl ether in H-ZSM-5 catalysts as a function of Si/Al ratio: a quasielastic neutron scattering study. Catal Sci Technol 10:4305–4320
Matam SK, Howe RF, Thetford A et al (2018) Room temperature methoxylation in zeolite H-ZSM-5: an operando DRIFTS/mass spectrometric study. Chem Commun 54:12875–12878
Matam SK, Nastase SAF, Logsdail AJ et al (2020) Methanol loading dependent methoxylation in zeolite H-ZSM-5. Chem Sci 11:6805–6814
Suwardiyanto S, Howe RF, Gibson EK et al (2017) An assessment of hydrocarbon species in the methanol-to-hydrocarbon reaction over a ZSM-5 catalyst. Faraday Discuss 197:447–472
Pinna RS, Rudić S, Parker SF et al (2018) The neutron guide upgrade of the TOSCA spectrometer. Nucl Instrum Methods Phys Res B 896:68–74
Parker SF, Lennon D, Albers PW (2011) Vibrational spectroscopy with neutrons: a review of new directions. Appl Spectrosc 65:1325–1341
Zachariou A, Hawkins AP, Lennon D et al (2019) Investigation of ZSM-5 catalysts for dimethylether conversion using inelastic neutron scattering. Appl Catal A Gen 569:1–7
Zachariou A, Hawkins AP, Collier P et al (2020) The effect of co-feeding methyl acetate on the H-ZSM5 catalysed methanol-to-hydrocarbons reaction. Top Catal 63:370–377
Torrie BH, Weng SX, Powell BM (1989) Structure of the α-phase of solid methanol. Mol Phys 67:575–581
Bordiga S, Regli L, Lamberti C et al (2005) FTIR adsorption studies of H2O and CH3OH in the isostructural H-SSZ-13 and H-SAPO-34: formation of H-bonded adducts and protonated clusters. J Phys Chem B 109:7724–7732
Campbell SM, Jiang XZ, Howe RF (1999) Methanol to hydrocarbons: spectroscopic studies and the significance of extra-framework aluminium. Microporous Mesoporous Mater 29:91–108
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, Oxford
Falk M, Whalley E (1961) Infrared spectra of methanol and deuterated methanols in gas, liquid, and solid phases. J Chem Phys 34:1554–1568
Allan A, McKean DC, Perchard JP et al (1971) Vibrational spectra of crystalline dimethyl ethers. Spectrochim Acta A Mol Spectrosc 27A:1409–1437
Corsaro C, Crupi V, Majolino D et al (2006) Inelastic neutron scattering study of water in hydrated LTA-type zeolites. J Phys Chem A 110:1190–1195
Li J (1996) Inelastic neutron scattering studies of hydrogen bonding in ices. J Chem Phys 105:6733–6755
Sears VF (1992) Neutron scattering lengths and cross sections. Neutron News 3:26–37
Nastase SAF, Cnudde P, Vanduyfhuys L et al (2020) Computational QM/MM investigation of the adsorption of MTH active species in H-Y and H-ZSM-5. ACS Catal 10:8904–8915
Tomkinson J, Parker SF, Lennon D (2010) No evidence for Evans’ holes in the A, B, C vibrational structure of potassium dihydrogen arsenate. J Chem Phys 133:034508
Lennon D, Zachariou A, Hawkins AP, Collier P, Parker SF (2018) STFC, ISIS Neutron Muon Source. https://doi.org/10.5286/ISIS.E.RB1810859
Lennon D, Collier P, Zachariou A, Hawkins AP, Collier P, Parker SF (2019) STFC, ISIS Neutron Muon Source. https://doi.org/10.5286/ISIS.E.RB1910561
Lennon D, Zachariou A, Howe RF, Hitchcock I, Hawkins A, Parker SF (2019) STFC, ISIS Neutron Muon Source. https://doi.org/10.5286/ISIS.E.RB1920589
Lennon D, Hawkins A, Zachariou A, Collier P, Parker SF, Howe RF (2018) STFC, ISIS Neutron Muon Source. https://doi.org/10.5286/ISIS.E.RB1820116
Acknowledgements
Johnson Matthey plc. is thanked for supplying the ZSM-5 zeolite and for financial support through the provision of industrial CASE studentships in partnership with the EPSRC (APH (EP/P510506/1, AZ (EP/N509176/1)). Experiments at the ISIS Neutron and Muon Source were made possible by beam time allocations from the Science and Technologies Facilities Council [35,36,37,38]. The resources and support provided by the UK Catalysis Hub via membership of the UK Catalysis Hub consortium and funded by EPSRC grants EP/R026815/1 and EP/R026939/1 are gratefully acknowledged. This research has been performed with the use of facilities and equipment at the Research Complex at Harwell; the authors are grateful to the Research Complex for this access and support.
Funding
EPSRC, Johnson Matthey (through iCase studentships).
Author information
Authors and Affiliations
Contributions
AZ (Data acquisition and analysis, first draft and editing of manuscript). APH (Neutron data acquisition). RFH (Validation and manuscript editing). PC (Industrial supervision and project conceptualisation). NB (NMR spectroscopy). JB (NMR spectroscopy). DL (Project management, supervision, validation and manuscript editing). SFP (Project management, supervision, validation and manuscript editing).
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zachariou, A., Hawkins, A.P., Howe, R.F. et al. A Spectroscopic Paradox: The Interaction of Methanol with ZSM-5 at Room Temperature. Top Catal 64, 672–684 (2021). https://doi.org/10.1007/s11244-021-01462-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01462-9