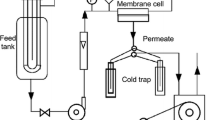
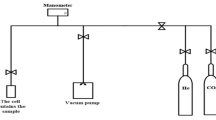
Abstract
The recovery of dimethylformamide (DMF) by pervaporation is less energy intensive and more economical than the traditional distillation method. High/pure silica zeolite is a typical organics perm-selective material for pervaporation membrane due to its hydrophobic nature, demonstrating great potential for recovering organic components from aqueous solutions. In this study, as an attempt to further enhance the membrane hydrophobicity, titanium and zirconium-substituted MEL type zeolite membranes (Ti-silicalite-2 and Zr-silicalite-2) were synthesized on the α-Al2O3 discs by a secondary growth method. X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) results confirmed the isomorphous substitution of the MEL framework by Ti and Zr atoms. The effects of isomorphous substitution, feed temperature and concentration on the DMF recovery performance were investigated via systematically designed pervaporation experiments. The fluxes and separation factors both increased with the isomorphous substitution of heteroatom, as well as increasing feed temperature and decreasing feed concentration. The Ti-silicalite-2 membrane exhibited a high separation factor of 6.4 with a total flux of 0.98 kg·m−2·h−1 for a 5 wt% DMF/water feed at 343 K.
Similar content being viewed by others
Abbreviations
- A:
-
effective membrane area [m2]
- d:
-
spacing between the planes in the atomic lattice [Å]
- J:
-
permeate flux [kg·m−2·h−1]
- Kf :
-
Freundlich constant [-]
- M:
-
weight of the permeate [kg]
- n:
-
Freundlich constant [-]
- p:
-
adsorbate pressure [mbar]
- qe :
-
equilibrium adsorption capacity [mmol·g−1]
- R2 :
-
correlation coefficient [-]
- t:
-
time [h]
- v:
-
unit cell volume [Å3]
- X:
-
mass fraction of feed [-]
- Y:
-
mass fraction of permeate [-]
- α:
-
separation factor [-]
- θ:
-
X-ray diffraction angle [°]
- λ:
-
wavelength[nm]
- BEA:
-
Beta-Type
- CHA:
-
chabazite
- DMF:
-
dimethylformamide
- FAU:
-
faujasite
- FT-IR:
-
Fourier transform infrared
- GC:
-
gas chromatography
- IGA:
-
intelligent gravimetric analyzer
- LTA:
-
Linde-Type A
- MEL:
-
Mobil eleven
- MFI:
-
Mobil five
- MOR:
-
mordenite
- MW:
-
microwave
- NaA:
-
Natrium A
- SEM:
-
scanning electron microscopy
- TBAOH:
-
tetrabutylammonium hydroxide
- TBOT:
-
tetrabutyl titanate
- TEOS:
-
tetraethylorthosilicate
- XRD:
-
X-ray diffraction
- ZBOT:
-
zirconium n-butoxide
References
S. Das, A. K. Banthia and B. Adhikari, Desalination, 197, 106 (2006).
S.-L. Wee, C.-T. Tye and S. Bhatia, Sep. Purif. Technol., 63, 500 (2008).
X. Feng and R. Y. M. Huang, Ind. Eng. Chem. Res., 36, 1048 (1997).
Q. Liu, R. D. Noble, J. L. Falconer and H. H. Funke, J. Membr. Sci., 117, 163 (1996).
P. Shao and R. Y. M. Huang, J. Membr. Sci., 287, 162 (2007).
A. Çalhan, S. Deniz, J. Romero and A. Hasanoğlu, Korean J. Chem. Eng., 36, 1489 (2019).
J. Wang, W. Zhang, W. Li and W. Xing, Korean J. Chem. Eng., 32, 1369 (2015).
Z. Wang, Q. Ge, J. Shao and Y. Yan, J. Am. Chem. Soc., 131, 6910 (2009).
J. Jiang, L. Wang, L. Peng, C. Cai, C. Zhang, X. Wang and X. Gu, J. Membr. Sci., 527, 51 (2017).
H. Kita, Membrane, 20, 169 (1995).
M. Torres, M. Gutiérrez, V. Mugica, M. Romero and L. López, Catal. Today, 166, 205 (2011).
S. Aguado, J. Gascon, D. Farrusseng, J. C. Jansen and F. Kapteijn, Micropor. Mesopor. Mater., 146, 69 (2011).
F. Zhang, L. Xu, N. Hu, N. Bu, R. Zhou and X. Chen, Sep. Purif. Technol., 129, 9 (2014).
Z. Chen, D. Yin, Y. Li, J. Yang, J. Lu, Y. Zhang and J. Wang, J. Membr. Sci., 369, 506 (2011).
D. Korelskiy, T. Leppäjärvi, H. Zhou and M. Grahn, J. Membr. Sci., 427, 381 (2013).
T. Bowen, J. Membr. Sci., 215, 235 (2003).
L. Chai, H. Li, X. Zheng, J. Wang, J. Yang, J. Lu, D. Yin and Y. Zhang, J. Membr. Sci., 491, 168 (2015).
W. Sun, X. Wang, J. Yang, J. Lu, H. Han, Y. Zhang and J. Wang, J. Membr. Sci., 335, 83 (2009).
V. Sebastian, J. Motuzas, R. W. J. Dirrix, R. A. Terpstra, R. Mallada and A. Julbe, Sep. Purif. Technol., 75, 249 (2010).
S. Li, V. A. Tuan, J. L. Falconer and R. D. Noble, Micropor. Mesopor. Mater., 58, 137 (2003).
P. Chen, X. Chen, X. Chen, Z. An and H. Kita, Chin. J. Chem., 27, 1692 (2009).
S. Li, V. A. Tuan, R. D. Noble and J. L. Falconer, AIChE J., 48, 269 (2002).
L. Song and L. V. C. Rees, Micropor. Mesopor. Mater., 35, 301 (2000).
N. Kosinov and E. J. M. Hensen, J. Membr. Sci., 447, 12 (2013).
Y. Cao, Q. Zhang, R. Xu and J. Zhong, Ion Exchange & Adsorption, 32, 423 (2016).
Q. G. Wu, H. Shao, J. Zhong, Q. Zhang and R. Xu, Modern Chem. Ind., 35, 46 (2015).
H. Shao, Y. Zhou, J. Zhong, Q. G. Wu, Q. Zhang and B. Z. Yang, J. Chem. Eng. Chinese U., 28, 965 (2014).
Q. G. Wu, H. Shao, J. Zhong, Q. Zhang and R. Xu, Modern Chem. Ind., 39, 94 (2019).
G. T. Kokotailo, P. Chu, S. L. Lawton and W.M. Meier, Nature, 275, 119 (1978).
Y. Cheng and S. Pan, Mater. Lett., 100, 289 (2013).
D. P. Serrano, M. A. Uguina, R. Sanz, E. Castillo, A. Rodríguez, P. Sánchez, Micropor. Mesopor. Mater., 69, 197 (2004).
Q. Wu, R. Xu, J. Li, Q. Zhang, J. Zhong, W. Huang and X. Gu, J. Porous Mat., 22, 1195 (2015).
H. M. F. Freundlich, Z. Phys.Chem., 57, 385 (1906).
J. G. Wijmans and R. W. Baker, J. Membr. Sci., 107, 1 (1995).
T. Bowen, J. Membr. Sci., 225, 165 (2003).
M. Nomura, T. Yamaguchi and S.-I. Nakao, J. Membr. Sci., 144, 161 (1998).
J. Z. Yang, Q. L. Liu and H. T. Wang, J. Membr. Sci., 291, 1 (2007).
V. A. Tuan, S. Li, J. L. Falconer and R. D. Noble, J. Membr. Sci., 196, 111 (2002).
H. Kosslick, V. A. Tuan, R. Fricke, C. Peuker, W. Pilz and W. Storek, J. Phys. Chem., 97, 796 (1993).
S. Li, V. A. Tuan, R. D. Noble and J. L. Falconer, Ind. Eng. Chem. Res., 40, 6165 (2001).
Acknowledgement
This work was supported by Natural Science Foundation of Jiangsu Province (BK20200982), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA530001), Advanced Catalysis and Green Manufacturing Collaborative Innovation Center, Changzhou University, A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and State Key Laboratory of Materials-Oriented Chemical Engineering.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Supporting Information
11814_2021_839_MOESM1_ESM.pdf
Preparation of heteroatom isomorphously substituted MEL zeolite membranes for pervaporation separation of dimethylformamide/water mixtures
Rights and permissions
About this article
Cite this article
Wu, Q., Xu, R., Shao, H. et al. Preparation of heteroatom isomorphously substituted MEL zeolite membranes for pervaporation separation of dimethylformamide/water mixtures. Korean J. Chem. Eng. 38, 2150–2156 (2021). https://doi.org/10.1007/s11814-021-0839-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0839-8