Abstract
A typical high-efficiency solar cell device needs the best lattice matching between different constituent layers to mitigate the open-circuit voltage loss. In the present work, the physical properties of CdS thin films are investigated where films with 100 nm thickness were fabricated on the different types of substrates viz. soda–lime glass, indium-doped tin oxide (ITO)- and fluorine-doped tin oxide (FTO)-coated glass substrates, and silicon wafer using electron beam evaporation. The X-ray diffraction patterns confirmed that deposited thin films showed cubic phase and had (111) as predominant orientation where the structural parameters were observed to be varied with nature of substrates. The ohmic behaviour of the CdS films was disclosed by current–voltage characteristics, whereas the scanning electron microscopy micrograph revealed the uniform deposition of the CdS films with the presence of round-shaped grains. The elemental analysis confirmed the CdS films deposition where the Cd/S weight percentage ratio was changed with nature of substrates. The direct energy band gap was observed in the 1.63–2.50 eV range for the films grown on different substrates. The investigated properties of thin CdS layers demonstrated that the selection of substrate (in terms of nature) during device fabrication plays a crucial role.
Similar content being viewed by others
1 Introduction
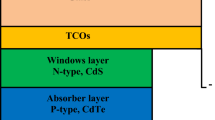
The increasing demand of energy in the era of cutting-edge technology and limited stock of fossil fuels have drawn kind attention for developing cost-effective and high-efficiency solar cell devices [1]. The silicon solar cell technology has been the champion ever since its invention while the thin film-based cost-effective technologies viz. CdTe, CIGS, organic, and perovskites have also come into existence with time which have their own merits and demerits. A solar cell device needs an optical window for ensuring the formation of an asymmetric junction with a corresponding absorber layer in order to derive the generated charge carriers towards the electrodes concerned [2]. For the CIGS and CdTe technologies, the CdS is a well-studied optical window material as it is having the best lattice matching with these absorber materials. Recently, the perovskite solar cell technology has come into existence and received extensive attention owing to low cost, high efficiency, and ease of fabrication procedure [3]. The high power conversion efficiency of the perovskite solar cells is achieved by using a mesoporous TiO2 electron transport layer (ETL) since the transport layers (viz. electron and hole transport layers) play an important role in the device performance. In such high-efficiency devices, the high-temperature annealing (> 450 °C) condition is required to crystallize the TiO2 films. However, it has been reported that the light-induced degradation of the solar cells is due to light-induced desorption of the oxygen and light-activated catalytic effect of TiO2, thus, the perovskite-based devices are facing stability problem. To reduce the degradation and enhance the stability, new materials for ETL have been proposed including ZnO, CdSe, CdS, SnO2, graphene, etc. [4,5,6,7].
Research is being pursued on CdS thin films due to their wide energy band gap (2.42 eV), high light sensitivity, low absorption coefficient, high transparency, and high electron affinity, which allow it in various optoelectronic applications [8,9,10,11]. It possesses n-type semiconducting nature owing to the existence of high sulphur vacancies and interstitial cadmium atoms, while the crystalline phase may be changed from cubic to hexagonal with air annealing at 300–350 °C, where the cubic phase of CdS is preferred for optoelectronic devices, and the hexagonal phase of CdS is suitable for solar cell applications [12,13,14,15]. The CdS thin films could be deposited by several physical and chemical routes based techniques which include chemical bath deposition [15], electron beam (e-beam) evaporation [16], pulsed laser deposition [17], sol–gel [18], electro-deposition [19], sputtering [20,21,22], atomic layer deposition [23], etc. Among these, the e-beam evaporation technique has key benefits compared to the others which include high purity and homogeneity of the grown films, control over the growth rate, etc. [24,25,26]. The films developed by this technique usually show high stoichiometry, stability, and durability. The e-beam evaporation technique maintains the homogeneity of films even at a lower thickness of the films. It also offers flexible doping possibility, least contamination, and highly adhesive and dense films [27] compared to other deposition techniques.
Since the properties of thin films are influenced by deposition techniques and respective parameters, nature of substrates, doping, annealing temperature and atmosphere, layer thickness, etc., therefore, extensive studies have been carried out on the CdS thin films [28,29,30,31,32,33,34,35,36,37]. Among these, the nature of substrate is also crucial factor as the used substrate not only provides mechanical support to thin films but it also plays a vital role in the modification of various physical properties. An ideal or typical substrate should inert and not react with the growing thin films. There should be the least thermal and lattice mismatching between the grown layer and substrate concerned.
The thin film growth process includes nucleation, coalescence, channels, holes, and continuous deposition [38] where underlying substrate induces different morphologies which is a consequence of atomic deposition on the surface of substrate concerned and relaxation of surface profile [39]. The major challenges in deposition of CdS thin films on different substrates include achievement of homogeneous and uniform deposition [40, 41], higher transmittance (in visible region) with large band gap, least lattice and thermal mismatch with the underneath substrates and selection of the substrates which uptake low moisture and release high moisture [42], etc.
The CdS films can be grown on different substrates viz. soda–lime glass, ITO, FTO, silicon, etc. The silicon is may be used as substrates for CdS films [41, 43, 44] and the crystalline silicon (c-Si) as p-type counterpart to n-type CdS in order to develop the heterostructure solar cells [45]. Despite of relatively higher lattice mismatch, the CdS films are widely grown on ITO and FTO substrates in fabrication of CdTe/CdS superstrate solar cell devices as these substrates efficiently work as transparent electrodes. Besides the above, the plastic foil, polymer (low-density polyethylene) [46], polyimide (PI) [42], quartz [41], Ti foils [47], polycarbonate (PC), polyethylene terephthalate (PET) [40], Si nanoporous pillar array (Si-NPA) [39], aluminium, platinum, molybdenum [48], etc. have also been used as substrates for the CdS thin films.
With consideration that the performance of CdTe/CdS solar cells can be improved by the use of enhanced quality CdS optical window where the properties of CdS films could be modified by different substrates, and consequently, the present article is devoted to the well-ordered study on microstructural and optoelectrical properties in correlation with the elemental properties of evaporated CdS thin films grown on different substrates viz. glass, ITO, FTO and Si wafer followed by undertaking microstructural and optical properties with substrate evolution to implicate as optical window or electron transport layer in the technologies concerned.
2 Experimental
The CdS powder with a purity of 5 N was procured from Sigma-Aldrich, and thin films with 100 nm thickness were deposited on soda–lime glass, and ITO- and FTO-coated substrates as well as on Si wafer (having < 111 > plane and n-type electrical conductivity) using an e-beam evaporation (Model-HHV box coater BC-300) process at room temperature. Prior to the deposition, all these substrates were washed by deionized water, acetone, isopropyl alcohol using an ultrasonication bath followed by desiccation. A schematic block diagram of e-beam evaporation method is shown in Fig. 1.
The evaporating CdS material (in pellet form) is placed in a graphite crucible and the substrates are kept fixed on the holder, which were rotated to get uniform deposition of the films. The vacuum chamber was evacuated up to 2 × 10–6 mbar by using a combination of diffusion and rotary pumps, then the CdS material was bombarded with high energy electron beam (having energy in range of 5–20 kV) emitted from an electron gun. The heating of the accelerated e-beam with evaporating CdS source material resulted into its evaporation. Consequently, the evaporants are moved towards substrates and condensed on these followed by films growth as per prevalent mechanism. An in-house quartz crystal monitor was used to measure the final films thickness as 100 nm whereas the growth rate was kept almost constant (5–6 Å/s).
2.1 Structural Analysis
The structural study of the deposited CdS films was carried out employing X-ray diffraction (XRD, Model-Rigaku Ultima-IV) using CuKα radiation (λ = 0.15406 nm), where these measurements were recorded in the angle of diffraction (2θ) range 20–80°. The inter-planar spacing (d) was determined by Bragg’s law and lattice parameter (a) of the cubic phase CdS films was determined by Eq. (1) [49].
where h, k, l are the Miller indices of the preferred diffraction peak in the XRD patterns.
The crystallite size (D) was evaluated by the Scherrer’s formula (Eq. (2)) while the internal strain (ε), dislocations density (δ), and number of crystallites per unit area (N) were evaluated using the following Eqs. (3–5) [49,50,51].
where λ is incident X-ray radiation’s wavelength, and θ is the Bragg’s diffraction angle. Also, k is known as the Scherrer’s constant with a value of 0.94, β is the full width at half maxima (FWHM), and t is the CdS film’s thickness.
2.2 Optoelectrical Analysis
The optical measurements were recorded in a wavelength range of 380–1100 nm employing Perkin Elmer LAMBDA UV–Vis. spectrophotometer. During the measurements of optical absorbance and transmittance spectra of the CdS films grown on different substrates, the underneath substrate concerned has been used as a reference sample in order to compensate and nullify the background data or baseline correction. Therefore, the obtained optical absorbance and transmittance data are only due to the grown CdS thin films. The energy band gap (Eg) was calculated using Eq. (6) [49, 51].
where hν is the photon energy, n is an integer having value 1 or 4 for allowing direct or indirect transitions, respectively, A is a constant which denotes the absorbance. The refractive index (n) is calculated by the Herve–Vandamme formula as given in Eq. (7) [52].
where constants A and B are having values as 13.6 eV and 3.4 eV, respectively. The Lorentz–Lorenz formula [53] was used to calculate the relative density of CdS films as given in Eq. (8).
where nf is the refractive index of the deposited CdS films, and nb is the refractive index of bulk CdS (having a value of 2.529). The optical response of CdS layers was studied by optical conductivity (σ0) as evaluated by Eq. (9) [9].
where c is the velocity of light. The real part (εr) and imaginary part (εi) of the complex dielectric constant (ε) could be calculated by Eqs. (10–11) [53, 54].
In order to perform the electrical measurements, the contacts on FTO and Si wafer substrates were made in sandwich structure (i.e. one contact on un-deposited substrate’s surface and other one on film’s surface) using adhesive silver paste (Sigma-Aldrich made). The measurements were undertaken using an Agilent B2901A source meter comprised of SMU software.
2.3 Surface Morphology and Elemental Analysis
The surface morphological analysis of the CdS films grown on the glass substrate was done by scanning electron microscopy (SEM, Model Raith), while elemental analysis for the CdS films grown on glass, ITO, and FTO substrates was carried out by the energy dispersive X-ray (EDX) spectroscopy.
3 Results and Discussion
3.1 Structural Analysis
The XRD patterns of the CdS films are depicted in Fig. 2 (a–d) wherein the sharp diffraction peak corresponding to (111) orientation is observed at 2θ positions of 26.54°, 26.52°, 26.64°, and 26.36° for CdS thin films grown on soda–lime glass, ITO- and FTO-coated glass substrates, and silicon wafer, respectively (JCPDS data file number 10–0454). The similar (111) preferred orientation is obtained for e-beam evaporated CdS films grown on different substrates [33]. The identified cubic (111) orientation of CdS films near 26° is in consistent with the earlier published reports where CdS films were developed on glass and Si wafer substrates [55, 56]. Another peak having (222) orientation is found at 54.62° for the CdS thin films deposited on FTO substrate.
In XRD patterns, ‘*’ and ‘#’ represent the peaks corresponding to used substrates. The intensity of (111)C preferred orientation for the films grown on the FTO substrate is higher as compared to the films grown on ITO and Si wafer while the maximum intensity is observed for the CdS thin films on the glass substrate which indicated the better crystallinity of films deposited on the glass and FTO substrates as compared to the others. The observed variation in intensity of the thin CdS films might be due to different adhesive nature of the CdS material on different substrates as well as mismatching in lattice and thermal coefficients of CdS material and underneath used substrate concerned [57]. The development of a cubic phase with the F \(\bar{4}\) 3 m space group is suitable for optoelectronic devices [14].
The structural parameters were calculated corresponding to the (111)C preferred orientation and are summarized in Table 1. The lattice constant (a) and inter-planar spacing (d) are found in the range of 5.78–5.83 Å and 3.34–3.37 Å, respectively, which agreed well with the standard JCPDS data. The lattice constant of CdS films grown on glass, ITO and FTO substrates is slightly lower than that of the standard lattice constant (5.82 Å) which might be possible due to the formation of sulphur vacancies in these films [58] as observed in the elemental analysis. The CdS films grown on Si wafer showed slightly higher lattice constant which may be due to the inclusion of impurity atoms [59]. The crystallite size (D) was found to be in range of 23–37 nm and observed maximum for the CdS films developed on FTO-coated glass substrate due to its lower FWHM which might be owing to the higher surface smoothening as compared to others [60], which indicated higher degree of nucleation, huge structural refinements, and greater crystalline quality [59, 61].
The internal strain (ε) is defined as the lattice disarrangement and found in the range (4.19–6.78) × 10−3, while the number of crystallites in the unit area (N) and dislocation density (δ) were observed to be changed with the nature of substrates due to alteration in respective crystallite size (D) and lattice constants of the material to the underlying substrates too [62, 63]. The observed XRD patterns and determined structural constants showed that the nature of substrates plays a crucial role in enhancing the structural properties of the deposited thin CdS films.
3.2 Optoelectrical Analysis
The transmittance and absorbance spectra of the developed thin CdS films are presented in Fig. 3. The CdS thin films show high absorbance in the 380–500 nm range of the visible region (Fig. 3a) as compared to the upper wavelength region owing to the existence of its conventional absorption in this range. It is found higher in lower wavelength range for all the substrates and later found to be decreased in the higher wavelength region after 500 nm for all films deposited on glass, ITO and FTO substrates while the films on silicon wafer showed an anomalous behaviour which might be due to light absorption by Si substrate owing to its relatively narrower/lower band gap. The absorbance is observed to be almost constant within the 600–1100 nm wavelength range for the films grown on the ITO and FTO substrates. As per Fig. 3(b), the transmittance (in the wavelength range of 500–1100 nm) is found more than 60% for CdS films on ITO- and FTO-coated glass substrates while it is lower for films on soda–lime glass and least for CdS films grown on Si wafer. A decrement in transmittance is observed near the absorption edge for ITO and FTO substrates which confirmed the good crystallinity [8]. The larger transmittance is found for the films grown on ITO and FTO substrates which reveal to the lower defect density on both the substrates [40], while the lower transmittance for glass and Si substrates is also possible due to higher packing density of these films [64].
The optical energy band gap (Eg) was evaluated from the optical absorption spectra using well-known Tauc’s relation (Eq. (6)) by extrapolating the straight line towards the energy axis at zero absorption coefficient as shown in Fig. 4. The linear nature of the Tauc’s plots confirmed direct transition and optical energy band gap is obtained as 2.25 eV, 2.33 eV, 2.50 eV, and 1.63 eV for films deposited on soda–lime glass, ITO-, FTO-coated substrates, and silicon wafer, respectively. The variation in optical energy band gap might be owing to the alteration in the stoichiometry of CdS films or change in atomic/weight percentage of Cd and S elements at different substrates, presence of impurities, variation in crystallinity and grain size, change in mobility and free carrier concentration, etc. [16, 65,66,67]. The band gap is proportional to strain as well as lattice constant of films whereas it has an inverse relationship with the crystallite size [68].
The CdS films grown on Si substrate show a relatively lower band gap (1.63 eV) as compared to the bulk counterpart (2.42 eV) and the films grown on other substrates as well which may be attributed to the less defined absorption edge which could lead to the formation of new level in the forbidden band gap [61]. The structural defects present in the films may also cause shrinkage in the band gap of films [69] and thus, the band gap is found lower. The calculated refractive index was found in the range of 2.51–2.88 and varied with the nature of the underlying substrates due to alteration in corresponding optical energy band gap might be ascribed to the variation in packing density of the grown films and changes in polarizability of ions in the films concerned [70]. The relative density is a dimensionless quantity that relates the refractive index of deposited films with the standard refractive index of bulk material concerned. The relative density is strongly dependent upon the crystallite size of films. In the present study, the relative density is found in range of 0.99–1.07 for the films grown on different substrates owing to corresponding changes in the crystallite size and crystallinity of CdS films. Lisco et al. [71] demonstrated almost similar behaviour of optical parameters for CdS films as a function of the deposition power. The real and imaginary components of the complex dielectric constant of films are related to the regional field distribution within the samples. The real (εr) and imaginary (εi) dielectric constants of CdS films are shown in Fig. 5 which are calculated using relations concerned [70]. It is evident from Fig. 5(a) that the real part exhibited almost similar behaviour for the films grown on soda–lime glass, ITO and FTO substrates while it is increased with photon energy for the films grown on Si wafer. The imaginary part (Fig. 5(b)) is directly related to the density of state within the forbidden energy gap of the semiconductor material [72]. It is found to be decreased slightly for the films grown on ITO and FTO substrates at lower energy regions and then increased, while decreased continuously for Si wafer, which might be due to the regional field distribution inside the samples and loss factor increase with photon energy [8].
The variation in optical conductivity (σ0) is presented in Fig. 6 (a) wherein σ0 increased with photon energy, which may be due to electrons excitation by the incident photon energy. It remained almost constant till hʋ ~ 2.00 eV and then increased because of absorption of the photon energy due to excitation of the electrons [73, 74].
The current–voltage (I–V) characteristics of CdS films deposited on FTO and Si wafer are presented in Fig. 6 (b) which reflected the linear dependency of current on voltage in the entire voltage range, demonstrating the ohmic nature of the films. Both the characteristics have almost coincided, and the current is slightly more for CdS thin films on FTO-coated glass substrates as compared to Si wafer, which may be due to the corresponding maximum crystallite size and decrease in grain boundaries [16]. The electrical resistivity is found more for CdS films deposited on Si wafer which may be due to the low mobility and reduction in carrier concentration. As per the elemental analysis of CdS films deposited on glass, ITO and FTO substrates, there is a sulphur vacancy and Cd richness nature in these films, which revealed to the n-type electrical conductivity of these semiconducting CdS thin films. Generally, for the thin films, the current attains zero value at zero voltage but, herein different behaviour is obtained at zero voltage and current, which revealed to slight capacitance behaviour of the thin films at the interface. The formation of ultrathin insulating oxide layer at the interface might be a possible reason for this capacitance behaviour where the impact of oxygen could be taken place due to the residual gases during the deposition.
3.3 Surface Morphology and Elemental Analysis
Surface morphological image of the CdS films deposited on glass substrate is shown in Fig. 7 (a) where the surface is found homogeneous, uniform and free from any defects. The film surface is densely packed with the presence of small round-shaped grains of similar size which revealed to the uniform nucleation throughout the substrate surface.
The grain size of CdS films grown on a glass substrate is close to 30 nm which is larger than that of the crystallite size obtained by the XRD data. Such variation in grain size and the crystallite size is possible since the grains are formed by agglomeration of smaller crystallites [75].
The EDX spectra of the thin CdS films deposited on glass, ITO and FTO are shown in Fig. 7 (b–d) which revealed to the presence of cadmium (Cd) and sulphur (S) elements in developed CdS thin films and confirmed the successful fabrication of CdS thin films on different substrates. Several other peaks corresponding to Si, Ca, Na, Mg, K, O, and Sn elements were also observed owing to the contribution from constituent elements of used underneath (glass, ITO and FTO) substrates. Similar elements/peaks are also observed by Ravichandran and Philominathan [76] for the CdS thin films which were grown employing spray pyrolysis method. The weight percentage of Cd:S in CdS films grown on glass, ITO and FTO substrates is listed in Table 2 which clearly demonstrated existence of the sulphur vacancies in these films. Similar sulphur vacancies were also observed in chemical bath deposited CdS films [77], where origin of these sulphur vacancies is attributed to the presence of oxygen in the CdS films [58, 77].
The above discussed physical properties to the CdS thin films grown on different substrates demonstrate that thin films are highly affected by the nature of underneath substrates. An anomalous behaviour is shown by the CdS thin films grown on silicon (< 111 > , n-type) wafer, which might be due to the band gap narrowness of the silicon substrate itself which influenced all the physical properties.
4 Conclusion
The present article meticulously demonstrated an impact of nature of underneath substrates on the properties of CdS layers grown on glass, ITO, FTO, and Si wafer by electron beam evaporation. The microstructural and optoelectrical properties of CdS films were assessed in order to seek their implications in solar cells. The structural analysis indicated the existence of cubic phase with (111) preferred orientation where the maximum crystallite size is found for the CdS films grown on FTO substrates. The conductivity is found better for the films developed on FTO-coated glass substrates. The SEM analysis demonstrated uniform, defect-free, and homogeneous surface of the CdS films deposited on the glass substrate. The EDX spectra confirmed the deposition of cadmium sulphide (CdS) films with Cd richness and sulphur vacancies. The transmittance for the films deposited on ITO and FTO substrates is found more than 60%. The experimental findings and analysed results viz. larger optical energy band gap, higher transmittance in visible region, the suitable crystallite size, higher electrical conductivity of CdS thin films grown on FTO substrates emphasize to utilize these as an effective optical window or buffer and electron transport layers to the solar cell technologies concerned.
References
C.R. Dong, Y. Wang, K. Zhang, H. Zeng, EnergyChem 2, 100026 (2020)
J. Nelson, The Physics of Solar Cells (Imperial College Press, London, 2002)
Z. Yu, A. Hagfeldt, L. Sun, Coord. Chem. Rev. 406, 213143 (2020)
M.A.K. Sheikh, R. Abdur, S. Singh, J.H. Kim, K.S. Min, J. Kim, J. Lee, Electronic. Mater. Lett. 14, 700 (2018)
W.S. Yang, B. Park, E.H. Jung, N.J. Jeon, Y.C. Kim, D.U. Lee, S.S. Shin, J. Seo, E.K. Kim, J.H. Noh, S.I. Seok, Science 356, 1376 (2017)
M.A. Green, E.D. Dunlop, J.H. Ebinger, M. Yoshita, N. Kopidakis, Y. Hishikawa, A.W.Y. Ho-Baillie, Prog. Photovolt: Res. Appl. 28, 3 (2020)
Y. Guo, J. Jiang, S. Zuo, F. Shi, J. Tao, Z. Hu, X. Hu, G. Hu, P. Yang, J. Chu, Sol. Energy Mater. Sol. Cells 178, 186 (2018)
M. Shaban, M. Mustafa, A.M.E. Sayed, Mater. Sci. Semicond. Process. 56, 329 (2016)
A.A. Galil, M.R. Balboul, A. Atta, I.S. Yahia, A. Sharaf, Phys. B 447, 35 (2014)
A.E. Alam, W.M. Cranton, I.M. Dharmadasa, J. Mater. Sci.: Mater. Electron. 30, 4580 (2019)
M. Shkir, I.M. Ashraf, A. Khan, M.T. Khan, A.M. El-Toni, S. AlFaify, Sens. Actuat. A Phys. 306, 111952 (2020)
S. Yilmaz, Appl. Surf. Sci. 357, 873 (2015)
A.A. Ziabari, F.E. Ghodsi, Sol. Energy Mater. Sol. Cells 105, 249 (2012)
J. Lee, Thin Solid Films 451–452, 170 (2004)
A. Ashok, G. Regmi, A. Romero-Núñez, M. Solis-López, S. Velumani, H. Castaneda, J. Mater. Sci.: Mater. Electron. 31, 7499 (2020)
S. Chander, M.S. Dhaka, Thin Solid Films 638, 179 (2017)
B. Ullrich, D.A. Flores, M. Bhowmick, Thin Solid Films 558, 24 (2014)
Y.A. Douri, Q. Khasawneh, S. Kiwan, U. Hashim, S.B.A. Hamid, A.H. Reshak, A. Bouhemadou, M. Ameri, R. Khenata, Energy Convers. Manage. 82, 238 (2014)
N. Nobari, M. Behboudnia, R. Maleki, Mater. Sci. Eng. B 224, 181 (2017)
C.T. Tsai, D.S. Chuu, G.L. Chen, S.L. Yang, J. Appl. Phys. 79, 9105 (1996)
J.W. Zhang, G. He, L. Zhou, H.S. Chen, X.S. Chen, X.F. Chen, B. Deng, J.G. Lv, Z.Q. Sun, J. Alloys Compd. 611, 253 (2014)
G. He, J. Liu, H. Chen, Y. Liu, Z. Sun, X. Chen, M. Liu, L. Zhang, J. Mater. Chem. C 2, 5299 (2014)
G. He, J. Gao, H. Chen, J. Cui, Z. Sun, X. Chen, A.C.S. Appl. Mater. Interfaces 6, 22013 (2014)
G. Laukaitis, D. Virbukas, Solid State Ionics 247–248, 41 (2013)
D. Virbukas, M. Sriubas, G. Laukaitis, Solid State Ionics 271, 98 (2015)
A. Purohit, S. Chander, M.S. Dhaka, Vacuum 153, 35 (2018)
R. Agarwal, Himanshu, S.L. Patel, M. Verma, S. Chander, C. Ameta, M.S. Dhaka, Opt. Mater. 116, 111033 (2021)
M. Shkir, M. Anis, S. Shafik, M.A. Manthrammel, M.A. Sayeed, M.S. Hamdy, S. AlFaify, Phys. E 118, 113955 (2020)
P. Kumar, N. Saxena, V. Gupta, J. Mater. Sci.: Mater. Electron. 31, 6755 (2020)
F. Göde, Optik 197, 163217 (2019)
R. Murugesan, S. Sivakumar, K. Karthik, P. Anandan, M. Haris, Curr. Appl. Phys. 19, 1136 (2019)
A. Kumar, V. Kumar, R. Chandra, Y.K. Gautam, Mater. Res. Exp. 6, 106448 (2019)
S. Chander, M.S. Dhaka, J. Mater. Sci.: Mater. Electron. 28, 6852 (2017)
A.A. Ojo, I.M. Dharmadasa, Sol. Energy 136, 10 (2016)
R. Zia, M. Riaz, Q. Ain, S. Anjum, Optik 127, 5407 (2016)
A. Slonopas, H. Ryan, B. Foley, Z. Sun, K. Sun, T. Globus, P. Norris, Mater. Sci. Semicond. Process. 52, 24 (2016)
H.Y. Cheng, J.S. Ma, C.H. Lu, J. Alloys Compd. 543, 84 (2012)
R. Agarwal, Himanshu, S.L. Patel, S. Chander, C. Ameta, M.S. Dhaka, Vacuum 177, 109347 (2020)
A.I. Oliva, R. Castro-Rodrıguez, O. Ceh, P. Bartolo Perez, F. Caballero-Briones, V. Sosa, Appl. Surf. Sci. 148, 42 (1999)
J.H. Lee, Thin Solid Films 515, 6089 (2007)
X.L. Tong, D.S. Jiang, W.B. Hu, Z.M. Liu, M.Z. Luo, Appl. Phys. A 84, 143 (2006)
M.G. Faraj, M.Z. Pakhuruddin, P. Taboada, J. Mater. Sci.: Mater. Electron. 28, 6628 (2017)
Y.A. Douri, M. Ameri, A. Bouhemadou, R. Khenata, Microsyst. Technol. 22, 2529 (2016)
Z.R. Khan, M. Zulfequar, M.S. Khan, Mater. Sci. Eng. B 174, 145 (2010)
V. Bilgin, E. Sarica, B. Demirselcuk, K. Ertürk, Phys. B 599, 412499 (2020)
M. Ouafi, B. Jaber, L. Laanab, Superlattice Microst. 129, 212 (2019)
M. Cao, L. Li, B.L. Zhang, J. Huang, K. Tang, H. Cao, Y. Sun, Y. Shen, J. Alloys Compd. 530, 81 (2012)
E. Fatas, R. Duo, P. Herrasti, F. Arjona, E. Gorcia-Camarero, J. Electrochem. Soc. 131, 2243 (1984)
A. Purohit, S. Chander, S.P. Nehra, C. Lal, M.S. Dhaka, Opt. Mater. 47, 345 (2015)
A. Purohit, S. Chander, S.P. Nehra, M.S. Dhaka, Phys. E 69, 342 (2015)
A. Purohit, S. Chander, A. Sharma, S.P. Nehra, M.S. Dhaka, Opt. Mater. 49, 51 (2015)
S. Chander, M.S. Dhaka, J. Mater. Sci.: Mater. Electron. 27, 11961 (2016)
F. Wooten, Optical Properties of Solids (Academic Press, New York, 1972)
B. Mahrov, G. Boschloo, A. Hagfeldt, L. Dloczik, T. Dittrich, Appl. Phys. Lett. 84, 5455 (2004)
H. Moualkia, S. Hariech, M.S. Aida, Thin Solid Films 518, 1259 (2009)
N.S. Das, P.K. Ghosh, M.K. Mitra, K.K. Chattopadhyay, Phys. E. 42, 2097 (2010)
D. Agrawal, D. Suthar, R. Agarwal, Himanshu, S.L. Patel, M.S. Dhaka. Phys. Lett. A 384, 126557 (2020)
K. Ravichandran, P. Philominathan, Appl. Surf. Sci. 255, 5736 (2009)
A. Goktas, A. Tumbul, Z. Aba, A. Kilic, F. Aslan, Opt. Mater. 107, 110073 (2020)
S. Chander, A. Purohit, S.L. Patel, M.S. Dhaka, Phys. E 89, 29 (2017)
J. Ke, S. Chen, L. Song, P. Zhang, X. Cao, B. Wang, R. Zhang, Superlatt. Microst. 146, 106671 (2020)
F. Mikailzade, H. Turkan, F. Onal, O. Karatas, S. Kazan, M. Zarbali, A. Goktas, A. Tumbul, Appl. Phys. A 126, 768 (2020)
M. Ahmed, A. Bakry, A. Qasem, H. Dalir, Opt. Mater. 113, 110866 (2021)
S. Hemathangam, G. Thanapathy, S. Muthukumaran, J. Mater. Sci.: Mater. Electron. 27, 6800 (2016)
Y.S. Lo, R.K. Choubey, W.C. Yu, W.T. Hsu, C.W. Lan, Thin Solid Films 520, 217 (2011)
S.L. Patel, S. Chander, A. Purohit, M.D. Kannan, M.S. Dhaka, J. Phys. Chem. Solids 123, 216 (2018)
R.S. Mane, S.J. Roh, O.S. Joo, C.D. Lokhande, S.H. Han, Electrochim. Acta 50, 2453 (2005)
A. Goktas, Phys. E 117, 113828 (2020)
S. Yılmaz, I. Polat, M. Tomakin, E. Bacaksiz, Appl. Phys. A 125, 67 (2019)
A. Goktas, I.H. Mutlu, J. Electron. Mater. 45, 5709 (2016)
F. Lisco, P.M. Kaminski, A. Abbas, J.W. Bowers, G. Claudio, M. Losurdo, J.M. Walls, Thin Solid Films 574, 43 (2015)
S. Chander, M.S. Dhaka, Mater. Chem. Phys. 185, 202 (2017)
F. Yakuphanoglu, A. Cukurovali, I. Yilmaz, Opt. Mater. 27, 1363 (2005)
A.N. Bakr, A.M. Funde, V.S. Waman, M.M. Kamble, R.R. Hawaldar, D.P. Amalnerkar, S.R. Gosavi, S.R. Jadkar, Pramana-J. Phys. 76, 519 (2011).
A. Goktas, Appl. Surf. Sci. 340, 151 (2015)
K. Ravichandran, P. Philominathan, Sol. Energy 82, 1062 (2008)
Q.Q. Liu, J.H. Shi, Z.Q. Li, D.W. Zhang, X.D. Li, Z. Sun, L.Y. Zhang, S.M. Huang, Phys. B 405, 4360 (2010)
Acknowledgements
The authors are grateful to the CSIR-CEERI, Pilani, for providing the facilities of EDX measurements and to the Microelectronics Research Center, Iowa State University, Ames, USA, for the SEM facility. A. Purohit acknowledges the University Grants Commission, New Delhi, for a financial assistantship (F.25-1/2013-14(BSR)/7-123/2007(BSR)).
Author information
Authors and Affiliations
Corresponding author
Additional information
Available online at http://link.springer.com/journal/40195.
Rights and permissions
About this article
Cite this article
Purohit, A., Himanshu, Patel, S.L. et al. Substrate Evolution to Microstructural and Optoelectrical Properties of Evaporated CdS Thin Films Correlated with Elemental Composition. Acta Metall. Sin. (Engl. Lett.) 34, 1307–1316 (2021). https://doi.org/10.1007/s40195-021-01266-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-021-01266-6