Abstract
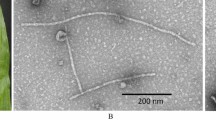
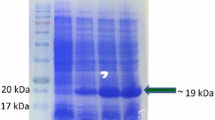
Onion yellow dwarf virus (OYDV) belonging to the genus Potyvirus, family Potyviridae, is one of the widely distributed viruses of Allium species worldwide. It causes dwarfing, yellow striping, crinkling and flaccidity of the leaves of onion and garlic. To see the occurrence and incidence of OYDV on Allium crop, an attempt was made to develop antibody based diagnostic assay which would be useful for routine indexing and screening of the germplasm. The total RNA was isolated from the symptomatic leaves of onion and the gene encoding coat protein (CP) was cloned. The nucleotide sequencing analysis of the cloned RT-PCR product revealed ~ 774 bp amplicon (OYDV CP) and it was further cloned in pET-28a ( +) expression vector which yielded ~ 30 kDa fusion protein with Histidine tag (His6BP). The expression of fusion CP was primarily checked on SDS–PAGE and further confirmed by Western blot. The His6BP-OYDV-CP was obtained in soluble state after purification and was used to immunize New Zealand white rabbit for the production of polyclonal antibody (PAb). The produced PAb against the purified fusion protein successfully detected OYDV from onion and garlic samples at 1:2000 dilutions in indirect-enzyme linked immunosorbent assay (DAC-ELISA). Thus, this study presents first report that Histidine tag (His6BP) fusion OYDV-CP based antibody production and its successful application in identification of virus free onion and garlic genotypes.




Similar content being viewed by others
References
Ahlawat YS, Varma A, Pant RP, Shukla A, Lockhart B (1996) Partial characterization of a badnavirus associated with citrus yellow mosaic disease in India. In Proceedings of 13th International Organisation of Citrus Virology. In: P Moreno, JV da Graca and LW Timmer (eds) Riveside California USA
Ahlawat YS, Varma A (1997) Serological detection of mixed viral infection in onion seed crop and possible measure for its management. Indian Phytopathol 50(1):137–140
Arya M, Baranwal VK, Ahlawat YS, Singh L (2006) RT–PCR detection and molecular characterization of onion yellow dwarf virus associated with garlic and onion. Curr Sci 91:1230–1234
Baghalian K, Kim OK, Natzuaki KT (2010) Molecular variability and genetic structure of the population of onion yellow dwarf virus infecting garlic in Iran. Virus Genes 41:282–291
Bagi F, Stojscaron V, Budakov D, ElSwaeh SM, GvozdanovićVarga J (2012) Effect of onion yellow dwarf virus (OYDV) on yield components of fall garlic (Allium sativum L.) in Serbia. Afr J Agric Res 7(15):2386–2390
Barg E, Lesemann DE, Vetten HJ, Green SK (1994) Identification, partial characterization, and distribution of viruses infecting Allium crops in South and South-East Asian countries. Acta Hortic 358:251–258
Barg E, Lesemann DE, Vetten HJ, Green SK (1997) Viruses of Alliums and their distribution in different Allium crops and geographical regions. Acta Hortic 433:607–616
Boscia D, Greif C, Gugerli P, Martelli GP, Walter B, Gonsalves D (1995) Nomenclature of grapevine leafroll-associated putative closteroviruses. Vitis 34(3):171–175
Bowden GA, Paredes AM, Georgiou G (1991) Structure and morphology of protein inclusion bodies in Escherichia coli. Biotechnology 8:725–730
Celli MG, Torrico AK, Kiehr M (2013) Striking differences in the biological and molecular properties of onion and garlic isolates of onion yellow dwarf virus. Arch Virol 158:1377–1382
Chodorska M, Paduch-Cichal E, Kalinowska E, Szyndel MS (2014) First report of onion yellow dwarf virus, garlic common latent virus and shallot latent virus on garlic in Poland. Dis Notes 98(6):858
Clark MF, Adams AN (1977) Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol 34(3):475–483
Clark MF, Bar-Joseph M (1984) Enzyme immunosorbent assays in plant virology. In: Maramorosch K, Koprowski H (eds) Methods in virology, vol 3. Academic Press, New York, pp 51–85
Diekmann M, Frison EA, Putter T (eds) (1994) Corporate Author: Food and Agriculture Organization (FAO), Rome, Italy. International Plant Genetic Resources Instit., (IPGRI), Rome, Italy, p 128. https://www.bioversityinternational.org/e-library/publications/detail/faoipgri-technical-guidelines-for-thesafe-movement-of-small-fruit-germplasm/
Djelouah K, Frasheri D, Onghia AMD (2002) Serological Diagnosis of Citrus psorosis virus and citrus tristeza virus using flower parts. Fifteenth IOCV Conference. Short communications. 363–365
El-Attar AK, Riad BY, Saad A, Soliman AM, Mazyad HM (2010) Expression of the coat protein gene of potato leafroll virus in Escherichia coli and development of polyclonal antibodies against recombinant coat protein. Arab J Biotechnol 13(1):85–98
FAOSTAT (2019) Onion production, area and productivity.http://www.fao.org/faostat/en/#data/QC. Accessed 1 March 2021
Gawande SJ, Chimote KP, Gurav VS, Gopal J (2013) Distribution and natural incidence of onion yellow dwarf virus (OYDV) on garlic and its related Allium species in India. Indian J Hortic 70(4):544–548
Ghosh DK, Ahlawat YS (1997a) Filamentous viruses associated with mosaic disease of garlic in India. Indian Phytopathol 50(2):266–276
Ghosh DK, Ahlawat YS, Gupta MD (1997b) Production of virus-free garlic (Allium sativum) plants by thremotherapy and meristem-tip culture. Ind J Agric Sci 67(12):591–594
Gibbs AJ, Varma A, Woods RD (1966) Viruses occurring in white clover (Trifolium repens) from permanent pastures in Britain. Ann Appl Biol 58:231
Hartley JL (2006) Cloning technologies for protein expression and purification. Curr Opin Biotechnol 17(4):359–366
Hoa NV, Ahlawat YS, Pant RP (2003) Partial characterization of onion yellow dwarf virus from onion in India. Indian Phytopathol 56(3):276–282
Hull R (2002) Matthews’ plant virology, 4th edn. Academic Press, New York
Kamo K, Jordan R, Guaragna MA (2010) Resistance to cucumber mosaic virus in gladiolus plants transformed with either a defective replicase or coat protein subgroup II gene from cucumber mosaic virus. Plant Cell Rep 29:695–704
Kapoor R, Mandal B, Paul PK, Chigurupati P, Jain RK (2014) Production of cocktail of polyclonal antibodies using bacterial expressed recombinant protein for multiple virus detection. J Virol Methods 196:7–14
Khan S, Jan AT, Mandal B, Haq QM (2012) Immunodiagnostics of cucumber mosaic virus using antisera developed against recombinant coat protein. Arch Phytopath Plant Prot 45(5):561–569
Khatabi B, He B, Hajimorad MR (2012) Diagnostic potential of polyclonal anti-bodies against bacterially expressed recombinant coat protein of alfalfa mosaic virus. Plant Dis 96:1352–1357
Kokane SB, Kokane AD, Misra P, Warghane AJ, Kumar P, Gubyad MG, Sharma AK, Biswas KK, Ghosh DK (2020) In-silico characterization and RNA-binding protein based polyclonal antibodies production for detection of citrus tristeza virus. Mol Cell Probes. https://doi.org/10.1016/j.mcp.2020.101654
Krystyna W, Ewa S, Wojciech S (2014) Prevalence of infections with onion yellow dwarf virus, leek yellow stripe virus and garlic common latent virus in plants from the genus Allium. Acta Scientiarum Polonorum 13(3):123–133
Kumar S, Baranwal VK, Joshi S, Arya M, Majumder S (2010) Simultaneous detection of mixed infection of onion yellow dwarf virus and an Allexivirus in RT-PCR for ensuring virus free onion bulbs. Indian J Virol 21(1):64–68
Kumari SG, Makkouk KM, Katul L, Vetten HJ (2001) Polyclonal antibodies to the bacterially expressed coat protein of Faba bean necrotic yellows virus. J Phytopathol 149(9):543–550
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lee SC, Chang YC (2008) Performances and application of antisera produced by recombinant capsid proteins of cymbidium mosaic virus and odontoglossum ring spot virus. Eur J Plant Pathol 122:297–306
Ling KS, Zhu HY, Gonsalves D (2004) Complete nucleotide sequence and genome organization of grapevine leafroll-associated virus 3, type member of the genus Ampelovirus. J Gen Virol 85:2099–2102
Majumder S, Johari S (2014) First report of onion yellow dwarf virus and garlic common latent virus infection in garlic from Nepal. J Plant Pathol. https://doi.org/10.4454/JPP.V96I4.012
Manglli A, Mohammed HS, Ali EL, Hussein A, Agosteo GE, Albanese G, Tomassoli L (2014) Molecular analysis of the 3′ terminal region of onion yellow dwarf virus from onion in southern Italy. Phytopathol Mediterr 53(3):258
Pramesh D, Singh P, Baranwal VK (2013) In vitro expression and purification of the coat protein gene of garlic common latent virus and its application in ELISA-based diagnostics. J Plant Pathol 95(2):385–389
Pringle CR (1998) Virus taxonomy-San Diego. Arch Virol 143:1449–1460
Rai R, Khurana SP, Kumar S, Gupta N, Baranwal VK (2018) Serological detection of grapevine leafroll-associated virus 4 in grapevine growing areas of India using polyclonal antiserum raised against the recombinant coat protein. Crop Prot 109:128–135
Raikhy G, Hallan V, Kulshrestha S, Zaidi AA (2007) Polyclonal antibodies to the coat protein of carnation etched ring virus expressed in bacterial system: production and use in immuno diagnosis. J Phytopathol 155:616–622
Rana T, Chandel V, Hallan V, Zaidi AA (2011) Expression of recombinant apple chlorotic leaf spot virus coat protein in heterologous system: production and use in immuno diagnosis. J Plant Biochem Biotechnol 20:138–141
Rani P, Pant RP, Jain RK (2010) Serological detection of cymbidium mosaic and odonto glossum rings pot viruses in orchids with polyclonal antibodies developed against their recombinant coat proteins. J Phytopathol 158:542–545
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Sevik MA, Akcura C (2013) Viruses occurring in onion crop in Amasya province, the major onion producing region in Turkey. Indian J Virol 24(1):78–81
Takaichi M, Yamamoto M, Nagakubo T, Oeda K (1998) Four garlic viruses identified by reverse transcription-polymerase chain reaction and their regional distribution in northern Japan. Plant Dis 82(6):694–698
Takaki F, Sanom T, Yamashita Y (2006) The complete nucleotide sequence of attenuated onion yellow dwarf virus: a natural potyvirus deletion mutant lacking the N-terminal 92 amino acids of HC-Pro. Arch Virol 151:1439–1445
Tiberini A, Mangano R, Micali G, Leo G, Manglli A, Tomassoli L, Albanese G (2018) Onion yellow dwarf virus∆∆ Ct-based relative quantification obtained by using real-time polymerase chain reaction in ‘Rossa di Tropea’onion. Eur J Plant Pathol 153(1):251–264
Tiberini A, Tomlinson J, Micali G, Fontana A, Albanese G, Tomassoli L (2019) Development of a reverse transcription-loop-mediated isothermal amplification (LAMP) assay for the rapid detection of onion yellow dwarf virus. J Virol Methods. https://doi.org/10.1016/j.jviromet.2019.113680
Van Dijk P (1993) Virus diseases of Allium species and prospects for their control. In International Symposium on Alliums for the Tropics. 358, pp 299–306
Verma RK, Mishra R, Petrov NM, Stoyanova M, Stoev A, Bakardjieva NV, Gaur RK (2015) Molecular characterization and recombination analysis of an Indian isolate of onion yellow dwarf virus. Eur J Plant Pathol 143:437–445
Walkey DG, Antill DN (1989) Agronomic evaluation of virusfree and virus-infected garlic (Allium sativum L.). J Hortic Sci 64(1):53–60
Acknowledgements
Authors are highly grateful to Head, Division of Plant Pathology, ICAR-Indian Agricultural Research Institute, New Delhi for encouragement and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Research involving in human and animal participants
This manuscript does not contain any experiment involving human or animal participants.
Rights and permissions
About this article
Cite this article
Kumar, R., Pant, R.P., Kapoor, S. et al. Development of polyclonal antibodies using bacterially expressed recombinant coat protein for the detection of Onion yellow dwarf virus (OYDV) and identification of virus free onion genotypes. 3 Biotech 11, 388 (2021). https://doi.org/10.1007/s13205-021-02921-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02921-6