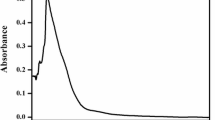
Abstract
Insecticides are extensively applied in crop production and sometimes at higher concentration than their recommended doses, which has become an environmental hazard. Sustainable agricultural practices highlight minimal application of insecticides at low concentration. Use of controlled release formulations (CRFs) of pesticides in which active compound is associated with inert materials has emerged as an appealing alternative. In this study, nanospheres of insecticide cartap hydrochloride were developed using chitosan (CS) in the presence of tripolyphosphate (TPP) as crosslinker with the help of ionic gelation method, for the delivery of cartap hydrochloride. The nanoformulations were characterized by field emission scanning electron microscope (FESEM), X-ray diffraction (XRD), and Fourier transform infrared (FTIR) spectra. The FESEM images revealed that the size of chitosan tripolyphosphate (CS-TPP) nanospheres (nps) was in range of 117.01–185.27 nm, whereas cartap hydrochloride entrapped chitosan tripolyphosphate (C-CS-TPP) nanospheres had a size of 163.50–276.74 nm. FTIR results confirmed loading of cartap hydrochloride into chitosan tripolyphosphate nanospheres. The nanospheres showed encapsulation efficiency of 86.1% and were stable for 30 days at ambient temperature. In vitro release kinetics of insecticide from nanospheres followed a non Fickian diffusion mechanism. These nanospheres can act as slow-release formulation for the delivery of insecticide to the target organisms over a period of time, which is effective as well as environmentally safe.








Similar content being viewed by others
References
Chaudhary ATMA, Kennedy IR (2005) Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biol Fertil Soils 39:219–227. https://doi.org/10.1007/s00374-003-0706-2
Pankratz FB, Doebel C, Farenhorst A, Goldsborough LG (2003) Interactions between algae (Selenastrum capricornutum) and pesticides: implications for managing constructed wetlands for pesticides removal. J Environ Sci Health B 38:147. https://doi.org/10.1081/PFC-120018445
Sun K, Weijie L, Lili L, Na W, Shunshan D (2013) Ecological risks assessment of organophosphorus pesticides on bloom of Microcystis wesenbergii. Int Biodeterior Biodegradation 77:98–105. https://doi.org/10.1016/j.ibiod.2012.11.010
Liu Y, Laks P, Heiden P (2002) Controlled release of biocides in solid wood. ii. efficacy against Trametes versicolor and Gloeophyllum trabeum wood decay fungi. J App Pol Sci 86:608–614. https://doi.org/10.1002/app.10897
Kumar M, Kumar SPR, Jeon BH, Thajuddin N (2014) Chlorpyrifos-induced changes in the antioxidants and fatty acid compositions of Chroococcus turgidus NTMS12. Lett Appl Microbiol 59(5):535–541. https://doi.org/10.1111/lam.12311
Wilkins RM (2004) Controlled release technology, agricultural. In: Seidel A. (ed.) Kirk Othmer Encyclopedia of chemical technology 5th Ed. New Jersey: John Wiley & Sons. https://doi.org/10.1002/0471238961.0107180907150518.a01
Chen H, Ruckenstein E (2014) Micellar structures in nanoparticle-multiblock copolymer complexes. Langmuir 30(13):3723–3728. https://doi.org/10.1021/la500450b
Chen H, Ruckenstein E (2013) Formation and degradation of multicomponent multicore micelles: insights from dissipative particle dynamics simulations Langmuir 29(18):5428–5434. https://doi.org/10.1021/la400033s
Chen H, Ruckenstein E (2012) Formation of complex colloidal particles: morphologies and mechanisms. Soft Matter 8(34):8911–8916. https://doi.org/10.1039/C2SM26035B
Peteu SF, Oancea F, Sicuia OA, Constantinescu F, Dinu S (2010) Responsive polymers for crop protection Polymers 2:229–251. https://doi.org/10.3390/polym2030229
Margulis GK, Magdassi S (2012) Nanotechnology: an advanced approach to the development of potent insecticides. In: Ishaaya I., Horowit Z.A.R. and Palli S.R. (eds.) Adv. Technolo. Manag. Insect Pests Dordrecht: Springer, 295–314. https://doi.org/10.1007/978-94-007-4497-4_15
Tomihata K, Ikada Y (1997) In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 18:567–575. https://doi.org/10.1016/s0142-9612(96)00167-6
Roberts GAF (1992) Preparation of chitin and chitosan. London Press, The Macmillan UK. https://doi.org/10.1007/978-1-349-11545-7_2
Kaur R, Goyal D, Agnihotri S (2021) Chitosan/PVA silver nanocomposite for butachlor removal: fabrication, characterization, adsorption mechanism and isotherms Carbohyd Polym 117906 https://doi.org/10.1016/j.carbpol.2021.117906
Agnihotri S, Mukherji S, Mukherji S (2012) Antimicrobial chitosan–PVA hydrogel as a nanoreactor and immobilizing matrix for silver nanoparticles. Appl Nanosci 2(3):179–188. https://doi.org/10.1007/s13204-012-0080-1
Agnihotri SA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release 100(1):5–28. https://doi.org/10.1016/j.jconrel.2004.08.010
Haque P, Mustafa A, Khan MA (2007) Effect of cross linking monomers on the physico-mechanical and degradation properties of photo grafted chitosan film. Carbohyd Polym 68:109–115. https://doi.org/10.1016/J.CARBPOL.2006.07.020
Gupta KC, Kumar MR (2000) Drug release behavior of beads and microgranules of chitosan. Biomaterials 21(11):1115–1119. https://doi.org/10.1016/S0142-9612(99)00263-X
Kalyaniwala K, Abhilash KPP, Victor PJ (2016) Cartap hydrochloride poisoning. Journal of the Association of Physicians of India 64. PMID: 27762121
Gan Q, Wang T (2007) Chitosan nanoparticle as protein delivery carrier—systematic examination of fabrication conditions for efficient loading and release. Colloids Surf B: Biointerfaces 59:24–34. https://doi.org/10.1016/j.colsurfb.2007.04.009
Gan Q, Wang T, Cochrane C, McCarron P (2005) Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery, Colloids Surf B 44:65–73. https://doi.org/10.1016/j.colsurfb.2005.06.001
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339(16):2693–2700. https://doi.org/10.1016/j.carres.2004.09.007
Prabaharan M, Mano JF (2005) Hydroxypropyl chitosan bearing –cyclodextrin cavities: synthesis and slow release of its inclusion complex with a model hydrophobic drug. Macromol Biosci 5:965–973. https://doi.org/10.1002/mabi.200500087
Grillo R et al (2010) Characterization of atrazine-loaded biodegradable poly (hydroxybutyrate-co-hydroxyvalerate) microspheres. J Polym Environ 18:26–32. https://doi.org/10.1007/s10924-009-0153-8
Higuchi T (1961) Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci 50:874–875. https://doi.org/10.1002/jps.2600501018
Korsmeyer RW et al (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35. https://doi.org/10.1016/0378-5173(83)90064-9
Costa P, Lobo JMS (2001) Modeling and comparison of dissolutions profiles. Eur J Pharm Sci 13:123–133. https://doi.org/10.1016/S0928-0987(01)00095-1
Xu Y, Du Y (2003) Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm 250(1):215–226. https://doi.org/10.1016/S0378-5173(02)00548-3
Vila A, Sánchez A, Janes K, Behrens I, Kissel T, Vila Jato JL, Alonso MJ (2004) Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm 57(1):123–131. https://doi.org/10.1016/j.ejpb.2003.09.006
Yang HC, Hon MH (2009) The effect of the molecular weight of chitosan nanoparticles and its application on drug delivery. Microchem J 92(1):87–91. https://doi.org/10.1016/j.microc.2009.02.001
Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MJ (2001) Chitosan nanoparticles as delivery systems for doxorubicin. J Controll Release 73(2–3):255–267. https://doi.org/10.1016/S0168-3659(01)00294-2
Cintia RM, Mariana G, Mônica P, Natalia B et al (2016) Nanoparticles based on chitosan as carriers for the combined herbicides Imazapic and Imazapyr. Sci Rep. https://doi.org/10.1038/srep19768
Yang Y, Cheng J, Garamus VM, Li N, Zou A (2018) Preparation of an environmentally friendly formulation of the insecticide nicotine hydrochloride through encapsulation in chitosan/tripolyphosphate nanoparticles J Agric Food Chem 66(5):1067–1074. https://doi.org/10.1021/acs.jafc.7b04147
Grillo R et al (2014) Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: An environmentally safer alternative for weed control. J Haz Mat 278:163–171. https://doi.org/10.1016/j.jhazmat.2014.05.079
Chauhan N, Dilbaghi N, Gopal M, Kumar R, Kim KH, Kumar S (2017) Development of chitosan nanocapsules for the controlled release of hexaconazole. Int J Biol Macromol 97:616–624. https://doi.org/10.1016/j.ijbiomac.2016.12.059
Pawlak A, Mucha M (2003) Thermo gravimetric and FTIR studies of chitosan blends, Thermochim. Acta 396:153–166. https://doi.org/10.1016/S0040-6031(02)00523-3
Dong Y, Xu C, Wang J, Wu Y, Wang M, Ruan Y (2002) Influence of degree of deacetylation on critical concentration of chitosan/dichlorocatic acid liquid crystalline solution. J Appl Polym Sci 83(6):1204–1208. https://doi.org/10.1002/app.2286
Jain A, Thakur K, Sharma G, Kush P, Jain UK (2016) Fabrication, characterization and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles Carbohydr. Polym 137:65–74. https://doi.org/10.1016/j.carbpol.2015.10.012
Hashad RA, Ishak RA, Fahmy S, Mansour S, Geneidi AS (2016) Methotrexate loading in chitosan nanoparticles at a novel pH: response surface modeling, optimization and characterization. Int J Biol Macromol 86:50–58. https://doi.org/10.1016/j.ijbiomac.2016.01.042
Krajewska B (2005) Membrane-based processes performed with use of chitin/chitosan materials. Sep Purif Technol 41(3):305–312. https://doi.org/10.1016/j.seppur.2004.03.019
Jayakumar R, Prabaharan M, Kumar PS, Nair S, Tamura H (2011) Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv 29(3):322–337. https://doi.org/10.1016/j.biotechadv.2011.01.005
Qin C, Li H, Xiao Q, Liu Y, Zhu J, Du Y (2006) Water solubility of chitosan and its antimicrobial activity. Carbohydr Polym 63(3):367–374. https://doi.org/10.1016/j.carbpol.2005.09.023
Dudhani AR, Kosaraju SL (2010) Bioadhesive chitosan nanoparticles: preparation and characterization. Carbohydr Polym 81:243–251. https://doi.org/10.1016/j.carbpol.2010.02.026
Wu Y, Yang W, Wang C, Hu J, Fu S (2005) Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int J Pharm 295(1–2):235–245. https://doi.org/10.1016/j.ijpharm.2005.01.042
Jia-hui Y, Yu-min D, Hua Z (1999) Blend films of chitosan–gelatin. J Wuhan Univ 45:440–444. https://doi.org/10.1007/BF02832288
Gomathi T, Sudha PN, Florence JAK, Venkatesan J, Anil S (2017) Fabrication of letrozole formulation using chitosan nanoparticles through ionic gelation method. Int J Biol Macromol 104:1820–1832. https://doi.org/10.1016/j.ijbiomac.2017.01.147
Lazaridou M, Christodoulou E, Nerantzaki M, Kostoglou M, Lambropoulou DA, Katsarou A, Bikiaris DN (2020) Formulation and in-vitro characterization of chitosan-nanoparticles loaded with the iron chelator deferoxamine mesylate (DFO). Pharmaceutics 12(3):238. https://doi.org/10.3390/pharmaceutics12030238
Rejinold NS, Chennazhi KP, Nair SV, Tamura H, Jayakumar R (2011) Biodegradable and thermo-sensitive chitosan-g-poly (N-vinylcaprolactam)nanoparticles as a 5-fluorouracil carrier. Carbohydr Polym 83:76–786. https://doi.org/10.1016/j.carbpol.2010.08.052
Xu Y, Du Y (2003) Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int J Pharm 250:215Y226. https://doi.org/10.1016/s0378-5173(02)00548-3
Mathew ME, Mohan JC, Manzoor K, Nair SV, Tamura H, Jayakumar R (2010) Folate conjugated carboxymethyl chitosan–manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Carbohydr Polym 80:442–448. https://doi.org/10.1016/j.carbpol.2009.11.047
Knaul JZ, Hudson SM, Creber KAM (1999) Improved mechanical properties of chitosan fibres. J Appl Polym Sci 72:1721Y1731. https://doi.org/10.1002/(SICI)1097-4628(19990624)72:13%3C1721:AID-APP8%3E3.0.CO;2-V
Wang X, Ma J, Wang Y, He B (2001) Structural characterization of phosphorylated chitosan and their applications as effective additives of calcium phosphate cements. Biomaterials. 22:2247Y2255. https://doi.org/10.1016/s0142-9612(00)00413-0.
Fajardo AR, Piai JF, Rubira AF, Muniz EC (2010) Time-and pH-dependent self-rearrangement of a swollen polymer network based on polyelectrolytes complexes of chitosan/chondroitin sulfate. Carbohyd Polym 80(934–943):4. https://doi.org/10.1016/j.carbpol.2010.01.009
Hao YM, Zhao FL, Li N, Yang YH, Li KA (2002) Studies on a high encapsulation of colchicine by a noisome system. Int J Pharm 244:73–80. https://doi.org/10.1016/s0378-5173(02)00301-0
Pendekal MS, Tegginamat PK (2013) Hybrid drug delivery system for oropharyngeal, cervical and colorectal cancer – in vitro and in vivo evaluation. Saudi Pharm J 21:177–186. https://doi.org/10.1016/j.jsps.2012.07.002
Papadimitriou S, Bikiaris D, Avgoustakis K, Karavas E, Georgarakis M (2008) Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohyd Polym 73:44–54. https://doi.org/10.1016/j.carbpol.2007.11.007
Li P, Wang Y, Peng Z, She F, Kong L (2011) Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohyd Polym 85:698–704. https://doi.org/10.1016/j.carbpol.2011.03.045
Subal CB (2006) Modelling of drug release: the Higuchi equation and its application. Pharmabiz.com
Dash S, Murthy PN, Nath L, Chowdhury P (2010) Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 67:217–223 (PMID: 20524422)
Singhvi G, Singh M (2011) In-vitro drug release characterization models. Int J Pharm Stud Res 2: 77–8. E-ISSN 2229–4619
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42. https://doi.org/10.1016/0168-3659(87)90035-6
Silva MS et al (2011) Paraquat-loaded alginate/chitosan nanoparticles: preparation, characterization and soil sorption studies. J Haz Mat 190:366–374. https://doi.org/10.1016/j.jhazmat.2011.03.057
Grillo R, Rosa AH, Fraceto LF (2014) Poly (ε -caprolactone) nanocapsules carrying the herbicide atrazine: effect of chitosan-coating agent on physico-chemical stability and herbicide release profile. Int J Environ Sci Technol 11:1691–1700. https://doi.org/10.1007/s13762-013-0358-1
Acknowledgements
The authors thank Head, Department of Biotechnology and Coordinator, Science and Technology Entrepreneurship Park (STEP), Thapar Institute of Engineering and Technology, Patiala, for infrastructure and laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, I., Goyal, D. & Agnihotri, S. Formulation of cartap hydrochloride crosslinked chitosan tripolyphosphate nanospheres and its characterization. Colloid Polym Sci 299, 1407–1418 (2021). https://doi.org/10.1007/s00396-021-04866-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-021-04866-x