Abstract
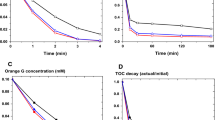
Diverse aromatic mediators have often been used to increase the degradation of organic pollutants by Fenton reaction (Fe2+ + H2O2 → Fe3+ + HO● + HO−). The presence of reducing mediators can minimize the accumulation of Fe3+ in solution, which leads to accelerated Fe2+ regeneration and enhances the generation of reactive oxygen species, such as the HO● radical, i.e., the strongest oxidant in Fenton-based processes. On the other hand, a few non-reducing compounds can be converted into reducing intermediates during reactions, in addition to assisting degradation processes. Therefore, the influence of aromatic mediators, reducers and non-reducers, on decolorization kinetics of Bismarck Brown Y (BBY) dye oxidized by Fenton processes (Fe2+/H2O2, Fe3+/H2O2) has been investigated herein by using data from a previous study that had been developed by the present research group. In general, the second-order reaction kinetic model was better fit to the experimental data. Improvements in apparent second-order rate constants (k2) were observed due to the presence of mediators. Reducers were more effective than non-reducers in increasing k2 values. In another kinetic model, BMG, maximum oxidation capacity was increased due to the presence of mediators. In a new set of experiments, a decrease in apparent activation energy was verified due to introducing mediators into reactions initially containing either Fe2+ or Fe3+ as catalysts to oxidize BBY at different temperatures. This indicates that the tested mediators, two reducers (hydroquinone, gallic acid) and a non-reducer (salicylic acid), can decrease the energy barrier to allow Fenton reaction to oxidize dyes more effectively.








Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and in https://link.springer.com/content/pdf/10.1007/s11356-017-0316-4.pdf.
Change history
05 September 2021
The equation 2 and Table 2 are now corrected.
References
Aguiar, A., & Ferraz, A. (2007). Fe3+- and Cu2+-reduction by phenol derivatives associated with Azure B degradation in Fenton-like reactions. Chemosphere, 66, 947–954. https://doi.org/10.1016/j.chemosphere.2006.05.067
Aguiar, A., Ferraz, A., Contreras, D., & Rodríguez, J. (2007). Mecanismo e aplicações da reação de Fenton assistida por compostos fenólicos. Química Nova, 30, 623–628. https://doi.org/10.1590/S0100-40422007000300023
Barreto, F., Santana, C. S., & Aguiar, A. (2016). Behavior of dihydroxybenzenes and gallic acid on the Fenton-based decolorization of dyes. Desalination and Water Treatment, 57, 431–439. https://doi.org/10.1080/19443994.2014.966333
Behnajady, M. A., Modirshahla, N., & Ghanbary, F. (2007). A kinetic model for the decolorization of C.I. Acid Yellow 23 by Fenton process. Journal of Hazardous Materials, 148, 98–102. https://doi.org/10.1016/j.jhazmat.2007.02.003
Chan, K. H., & Chu, W. (2003). Modeling the reaction kinetics of Fenton’s process on the removal of atrazine. Chemosphere, 51, 305–311. https://doi.org/10.1016/S0045-6535(02)00812-3
Chen, F., Ma, W., He, J., & Zhao, J. (2002). Fenton degradation of malachite green catalyzed by aromatic additives. Journal of Physical Chemistry A, 106, 9485–9490. https://doi.org/10.1021/jp0144350
Christoforidis, K. C., Vasiliadou, I. A., Louloudi, M., & Deligiannakis, Y. (2018). Gallic acid mediated oxidation of pentachlorophenol by the Fenton reaction under mild oxidative conditions. Journal of Chemical Technology and Biotechnology, 93, 1601–1610. https://doi.org/10.1002/jctb.5529
Değermenci, N., & Akyol, K. (2020). Decolorization of the Reactive Blue 19 from aqueous solutions with the Fenton oxidation process and modeling with deep neural networks. Water, Air, & Soil Pollution, 231, 72. https://doi.org/10.1007/s11270-020-4402-8
Devi, L. G., Raju, K. S. A., Kumar, S. G., & Rajashekhar, K. E. (2011). Photodegradation of di azo dye Bismarck Brown by advanced photo-Fenton process: Influence of inorganic anions and evaluation of recycling efficiency of iron powder. Journal of the Taiwan Institute of Chemical Engineers, 42, 341–349. https://doi.org/10.1016/j.jtice.2010.05.010
Ertugay, N., & Acar, F. N. (2017). Removal of COD and color from Direct Blue 71 azo dye wastewater by Fenton’s oxidation: Kinetic study. Arabian Journal of Chemistry, 10, S1158–S1163. https://doi.org/10.1016/j.arabjc.2013.02.009
Khan, N., Bhatti, H. N., Iqbal, M., & Nazir, A. (2019). Decolorization of Basic Turquise Blue X-GB and Basic Blue X-GRRL by the Fenton’s process and its kinetics. Zeitschrift Für Physikalische Chemie, 233, 361–373. https://doi.org/10.1515/zpch-2018-1194
Lee, C., & Yoon, J. (2004). Temperature dependence of hydroxyl radical formation in the hv/Fe3+/H2O2 and Fe3+/H2O2 systems. Chemosphere, 56, 923–934. https://doi.org/10.1016/S1383-5866(02)00200-9
Levenspiel, O. (1998). Chemical Reaction Engineering. Wiley.
Li, T., Zhao, Z., Wang, Q., Xie, P., & Ma, J. (2016). Strongly enhanced Fenton degradation of organic pollutants by cysteine: An aliphatic amino acid accelerator outweighs hydroquinone analogues. Water Research, 105, 479–486. https://doi.org/10.1016/j.watres.2016.09.019
Luo, L., Yao, Y., Gong, F., Huang, Z., Lu, W., Chen, W., & Zhang, L. (2016). Drastic enhancement on Fenton oxidation of organic contaminants by accelerating Fe(III)/Fe(II) cycle with L-cysteine. RSC Advances, 6, 47661–47668. https://doi.org/10.1039/C6RA07091D
Ma, X. H., Zhao, L., & Dong, Y. H. (2020). Oxidation degradation of 2,2′,5-trichlorodiphenyl in a chelating agent enhanced Fenton reaction: Influencing factors, products, and pathways. Chemosphere, 246, 125849. https://doi.org/10.1016/j.chemosphere.2020.125849
Malik, P. K., & Saha, S. K. (2003). Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst. Separation and Purification Technology, 31, 241–250. https://doi.org/10.1016/S1383-5866(02)00200-9
Nidheesh, P. V., Gandhimathi, R., & Ramesh, S. T. (2013). Degradation of dyes from aqueous solution by Fenton processes: A review. Environmental Science and Pollution Research, 20, 2099–2132. https://doi.org/10.1007/s11356-012-1385-z
Papoutsakis, S., Pulgarin, C., Oller, I., Sánchez-Moreno, R., & Malato, S. (2016). Enhancement of the Fenton and photo-Fenton processes by components found in wastewater from the industrial processing of natural products: The possibilities of cork boiling wastewater reuse. Chemical Engineering Journal, 304, 890–896. https://doi.org/10.1016/j.cej.2016.07.021
Qin, Y., Song, F., Ai, X., Zhang, P., & Zhang, L. (2015). Protocatechuic acid promoted alachlor degradation in Fe(III)/H2O2 Fenton system. Environmental Science & Technology, 49, 7948–7956. https://doi.org/10.1021/es506110w
Ramos, M. D. N., Cláudio, C. C., Rezende, P. H. V., Santos L. A., Cabral, L. P., Mesquita, P. L., & Aguiar, A. (2020a). Análise crítica das características de efluentes industriais do setor têxtil no Brasil. Revista Virtual de Quimica, 12, 913–929. https://doi.org/10.21577/1984-6835.20200073
Ramos, M. D. N., Sousa, L. A., & Aguiar, A. (2020b). Effect of cysteine using Fenton processes on decolorizing different dyes: A kinetic study. Environmental Technology. https://doi.org/10.1080/09593330.2020.1776402
Romero, R., Contreras, D., Segura, C., Schwederski, B., & Kaim, W. (2016). Hydroxyl radical production by a heterogeneous Fenton reaction supported in insoluble tannin from bark of Pinus radiata. Environmental Science and Pollution Research., 24, 6135–6142. https://doi.org/10.1007/s11356-016-7532-1
Santana, C. S., & Aguiar, A. (2015). Effect of biological mediator, 3-hydroxyanthranilic acid, in dye decolorization by Fenton processes. International Biodeterioration & Biodegradation, 104, 1–7. https://doi.org/10.1016/j.ibiod.2015.05.007
Santana, C. S., & Aguiar, A. (2016). Effect of lignin-derived methoxyphenols in dye decolorization by Fenton systems. Water, Air, & Soil Pollution, 227, 48. https://doi.org/10.1007/s11270-015-2703-0
Santana, C. S., Velloso, C. C. V., & Aguiar, A. (2019). Estudo cinético da descoloração do azocorante alaranjado de metila por processos Fenton na presença de di-hidroxibenzenos e ácido gálico. Revista Virtual de Química, 11, 104–114. https://doi.org/10.21577/1984-6835.20190008
Santana, C. S., Velloso, C. C. V., & Aguiar, A. (2019). Um estudo cinético sobre a influência de mediadores fenólicos na descoloração de diferentes corantes por sistemas Fenton. Química Nova, 42, 149–155. https://doi.org/10.21577/0100-4042.20170316
Santana, C. S., Ramos, M. D. N., Velloso, C. C. V., & Aguiar, A. (2019c). Kinetic evaluation of dye decolorization by Fenton processes in the presence of 3-hydroxyanthranilic acid. International Journal of Environmental Research and Public Health, 16, 1602. https://doi.org/10.3390/ijerph16091602
Silva, B. C., Perini, J. A. L., & Nogueira, R. F. P. (2017). Influence of dihydroxybenzenes on paracetamol and ciprofloxacin degradation and iron(III) reduction in Fenton processes. Environmental Science and Pollution Research, 24, 6157–6164. https://doi.org/10.1007/s11356-016-6402-1
Sousa, J. L., & Aguiar, A. (2017). Influence of aromatic additives on Bismarck Brown Y dye color removal treatment by Fenton processes. Environmental Science and Pollution Research, 24, 26734–26743. https://doi.org/10.1007/s11356-017-0316-4
Tang, D., Zhang, G., Wang, Y., Chen, F., & Ma, J. (2019). The reactivity and pathway of Fenton reactions driven by hydroxybenzoic acids: The effect of hydroxylation. Environmental Science: Water Research & Technology, 5, 1507–1514. https://doi.org/10.1039/c9ew00250b
Tkaczyk, A., Mitrowska, K., & Posyniak, A. (2020). Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Science of the Total Environment, 717, 137222. https://doi.org/10.1016/j.scitotenv.2020.137222
Torrades, F., García-Hortal, J. A., & García-Montaño, J. (2015). Mineralization of hetero bi-functional reactive dye in aqueous solution by Fenton and photo-Fenton reactions. Environmental Technology, 36, 2035–2042. https://doi.org/10.1080/09593330.2015.1019931
Wu, D., Feng, Y., & Ma, L. (2013). Oxidation of azo dyes by H2O2 in presence of natural pyrite. Water Air & Soil Pollution, 224, 1407.
Xavier, S., Gandhimathi, R., Nidheesh, P. V., & Ramesh, S. T. (2013). Comparison of homogeneous and heterogeneous Fenton processes for the removal of reactive dye Magenta MB from aqueous solution. Desalination and Water Treatment, 53, 109–118. https://doi.org/10.1080/19443994.2013.844083
Xiao, J., Wang, C., & Liu, H. (2020). Fenton-like degradation of dimethyl phthalate enhanced by quinone species. Journal of Hazardous Materials, 382, 121007. https://doi.org/10.1016/j.jhazmat.2019.121007
Zazo, J. A., Pliego, G., Blasco, S., Casas, J. A., & Rodriguez, J. J. (2011). Intensification of the Fenton process by increasing the temperature. Industrial & Engineering Chemistry Research, 50, 866–870. https://doi.org/10.1021/ie101963k
Zhang, M., Dong, H., Zhao, L., Wang, D., & Meng, D. (2019). A review on Fenton process for organic wastewater treatment based on optimization perspective. Science of the Total Environment, 670, 110–121. https://doi.org/10.1016/j.scitotenv.2019.03.180
Funding
This study was supported by Brazilian Agencies for Scientific and Technological Development: Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Fapemig, project number APQ-01898–17), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Ensino Superior (CAPES).
Author information
Authors and Affiliations
Contributions
Juan P. P. Lima, Carlos H. B. Tabelini, Márcio D. N. Ramos: experimental work; formal analysis; writing—original draft; André Aguiar: conceptualization; funding acquisition; project administration; supervision; writing—review and editing; visualization.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lima, J.P.P., Tabelini, C.H.B., Ramos, M.D.N. et al. Kinetic Evaluation of Bismarck Brown Y Azo Dye Oxidation by Fenton Processes in the Presence of Aromatic Mediators. Water Air Soil Pollut 232, 321 (2021). https://doi.org/10.1007/s11270-021-05258-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05258-1