Abstract
Objective
Type 2 diabetes (T2D) is one of the centenarian metabolic disorders and is considered as a stellar and leading health issue worldwide. According to the International Diabetes Federation (IDF) Diabetes Atlas and National Diabetes Statistics, the number of diabetic patients will increase at an exponential rate from 463 to 700 million by the year 2045. Thus, there is a great need for therapies targeting functions that can help in maintaining the homeostasis of glucose levels and improving insulin sensitivity. 5′ adenosine monophosphate-activated protein kinase (AMPK) activation, by various direct and indirect factors, might help to overcome the hurdles (like insulin resistance) associated with the conventional approach.
Materials and results
A thorough review and analysis was conducted using various database including MEDLINE and EMBASE databases, with Google scholar using various keywords. This extensive review concluded that various drugs (plant-based, synthetic indirect/direct activators) are available, showing tremendous potential in maintaining the homeostasis of glucose and lipid metabolism, without causing insulin resistance, and improving insulin sensitivity. Moreover, these drugs have an effect against diabetes and are therapeutically beneficial in the treatment of diabetes-associated complications (neuropathy and nephropathy) via mechanism involving inhibition of nuclear translocation of SMAD4 (SMAD family member) expression and association with peripheral nociceptive neurons mediated by AMPK.
Conclusion
From the available information, it may be concluded that various indirect/direct activators show tremendous potential in maintaining the homeostasis of glucose and lipid metabolism, without resulting in insulin resistance, and may improve insulin sensitivity, as well. Therefore, in a nut shell, it may be concluded that the regulation of APMK functions by various direct/indirect activators may bring promising results. These activators may emerge as a novel therapy in diabetes and its associated complications.




Similar content being viewed by others
References
IDF DIABETES ATLAS. 9th ed. (2019). https://diabetesatlas.org/data/en/world/. Accessed 22 Mar
Gupta A, Behl T, Sehgal A, Bhatia S, Jaglan D, Bungau S. Therapeutic potential of Nrf-2 pathway in the treatment of diabetic neuropathy and nephropathy. Mol Biol Rep. 2021;22:1–4.
CDC. U.S. Department of Health and Human Service. Center for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html; https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 28 Jul
Gupta A, Behl T, Sehgal A, Bhardwaj S, Singh S, Sharma N, Hafeez A. Exploring the recent molecular targets for diabetes and associated complications. Mol Biol Rep. 2021;24:1–7.
Gupta A, Behl T, Panichayupakaranan P. A review of phytochemistry and pharmacology profile of Juglans regia. Obes Med. 2019;16: 100142. https://doi.org/10.1016/j.obmed.2019.100142.
Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2016;61:187–96. https://doi.org/10.1016/j.survophthal.2015.06.001.
Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B, American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. https://doi.org/10.2337/dc08-9025.
Gupta A, Behl T, Sachdeva M. Key milestones in the diabetes research: a comprehensive update. Obes Med. 2020;17: 100183. https://doi.org/10.1016/j.obmed.2020.100183.
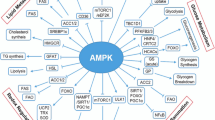
Mihaylova MM, Shaw RJ. The AMP-activated protein kinase (AMPK) signaling pathway coordinates cell growth, autophagy, & metabolism. Nat Cell Biol. 2011;13:1016–23. https://doi.org/10.1038/ncb2329.
Hardie DG, Ross FA, Hawley SA. AMPK—a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. https://doi.org/10.1038/nrm3311.
Popa AR, Bungau S, Vesa CM, Bondar AC, Pantis C, Maghiar O, Dimulescu IA, Cseppento DCN, Rus M. Evaluating the efficacy of the treatment with benfotiamine and alpha-lipoic acid in distal symmetric painful diabetic polyneuropathy. Rev Chim. 2019;70:3108–14.
Vesa CM, Popa L, Popa AR, Rus M, Zaha AA, Bungau S, Tit DM, Aron RAC, Zaha DC. Current data regarding the relationship between type 2 diabetes mellitus and cardiovascular risk factors. Diagnostics. 2020. https://doi.org/10.3390/diagnostics10050314.
Ford RJ, Fullerton MD, Pinkosky SL, Day EA, Scott JW, Oakhill JS, Bujak AL, Smith BK, Crane JD, Blumer RM, et al. Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem J. 2015;468:125–32. https://doi.org/10.1042/bj20150125.
Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–66. https://doi.org/10.1016/j.cmet.2014.09.018.
Greenfield JR, Chisholm DJ, Department of Endocrinology. Thiazolidinediones—mechanisms of action. Aust Prescr. 2004;27:67–70. https://doi.org/10.18773/austprescr.2004.059.
Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond). 2008;32:S7–12. https://doi.org/10.1038/ijo.2008.116.
Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66:789–800. https://doi.org/10.1016/j.molcel.2017.05.032.
Kazgan N, Williams T, Forsberg LJ, Brenman JE. Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase. Mol Biol Cell. 2010;21:3433–42. https://doi.org/10.1091/mbc.E10-04-0347.
Shrikanth CB, Nandini CD. AMPK in microvascular complications of diabetes and the beneficial effects of AMPK activators from plants. Phytomedicine. 2020;73: 152808. https://doi.org/10.1016/j.phymed.2018.12.031.
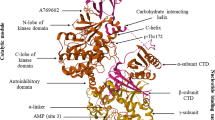
Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–84. https://doi.org/10.1172/jci200419874.
Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–51. https://doi.org/10.1038/nrd1344.
Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–72. https://doi.org/10.1172/jci67227.
Hardie DG, Carling D, Sim ATR. The AMP-activated protein kinase: a multisubstrate regulator of lipid metabolism. Trends Biochem Sci. 1989;14:20–3. https://doi.org/10.1016/0968-0004(89)90084-4.
Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–87. https://doi.org/10.1074/jbc.271.44.27879.
Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48: e245. https://doi.org/10.1038/emm.2016.81.
O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–55. https://doi.org/10.1038/nature11862.
O’Neill HM, Holloway GP, Steinberg GR. AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol. 2013;366:135–51. https://doi.org/10.1016/j.mce.2012.06.019.
Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget. 2015;6:7365–78. https://doi.org/10.18632/oncotarget.3629.
Chen W, Lu Y, Chen G, Huang S. Molecular evidence of cryptotanshinone for treatment and prevention of human cancer. Anti-Cancer Agents Med Chem. 2013;13(7):979–87. https://doi.org/10.2174/18715206113139990115 (PMID: 23272908; PMCID: PMC3625674).
Deng S, Wong CK, Lai HC, Wong AS. Ginsenoside-Rb1 targets chemotherapy-resistant ovarian cancer stem cells via simultaneous inhibition of Wnt/β-catenin signaling and epithelial-to-mesenchymal transition. Oncotarget. 2017;8(16):25897. https://doi.org/10.18632/oncotarget.13071 (PMCID:PMC5432225).
Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem. 2008;283:9187–95. https://doi.org/10.1074/jbc.M708934200.
Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J. 2008;409:449–59. https://doi.org/10.1042/BJ20071114.
Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–16. https://doi.org/10.1016/j.cmet.2006.05.005.
Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713–23. https://doi.org/10.1002/hep.26672.
Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2010;1804:581–91. https://doi.org/10.1016/j.bbapap.2009.09.012.
Sharpe LJ, Brown AJ. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J Biol Chem. 2013;288:18707–15. https://doi.org/10.1074/jbc.R113.479808.
Yang X, Mudgett J, Bou-About G, Champy MF, Jacobs H, Monassier L, Pavlovic G, Sorg T, Herault Y, Petit-Demoulière B, et al. Physiological expression of AMPKγ2RG mutation causes Wolff-Parkinson-White syndrome and induces kidney injury in mice. J Biol Chem. 2016;291:23428–39. https://doi.org/10.1074/jbc.M116.738591.
Shirwany NA, Zou MH. AMPK in cardiovascular health and disease. Acta Pharmacol Sin. 2010;31:1075–84. https://doi.org/10.1038/aps.2010.139.
Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191–200. https://doi.org/10.5114/aoms.2013.33181.
Resta N, Pierannunzio D, Lenato GM, Stella A, Capocaccia R, Bagnulo R, Lastella P, Susca FC, Bozzao C, Loconte DC, et al. Cancer risk associated with STK11/LKB1 germline mutations in Peutz-Jeghers syndrome patients: results of an Italian multicenter study. Dig Liver Dis. 2013;45:606–11. https://doi.org/10.1016/j.dld.2012.12.018.
Faubert B, Vincent EE, Poffenberger MC, Jones RG. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett. 2015;356:165–70. https://doi.org/10.1016/j.canlet.2014.01.018.
Wang Z, Wang N, Liu P, Xie X. AMPK and cancer. In: Cordero MD, Viollet B, editors. AMP-activated protein kinase. Cham: Springer International Publishing; 2016. p. 203–26. https://doi.org/10.1007/978-3-319-43589-3_9.
Hardie DG, Frenguelli BG. A neural protection racket: AMPK and the GABA(B) receptor. Neuron. 2007;53:159–62. https://doi.org/10.1016/j.neuron.2007.01.004.
Gailite I, Aerne BL, Tapon N. Differential control of Yorkie activity by LKB1/AMPK and the Hippo/Warts cascade in the central nervous system. Proc Natl Acad Sci USA. 2015;112:E5169–78. https://doi.org/10.1073/pnas.1505512112.
Rosso P, Fioramonti M, Fracassi A, Marangoni M, Taglietti V, Siteni S, Segatto M. AMPK in the central nervous system: physiological roles and pathological implications. Res Rep Biol. 2016;7:1–13. https://doi.org/10.2147/RRB.S90858.
Zhao J, Miyamoto S, You YH, Sharma K. AMP-activated protein kinase (AMPK) activation inhibits nuclear translocation of Smad4 in mesangial cells and diabetic kidneys. Am J Physiol-Renal Physiol. 2015;308(10):F1167–77.
Asiedu MN, Dussor G, Price TJ. Targeting AMPK for the alleviation of pathological pain. In: AMP-activated protein kinase. Cham: Springer; 2016. p. 257–85.
Popa AR, Fratila O, Rus M, Aron RAC, Vesa CM, Pantis C, Diaconu CC, Bratu O, Bungau S, Nemeth S. Risk factors for adiposity in the urban population and influence on the prevalence of overweight and obesity. Exp Ther Med. 2020;20:129–33. https://doi.org/10.3892/etm.2020.8662.
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. Nutrient excess in AMPK downregulation and insulin resistance. J Endocrinol Diabetes Obes. 2013;1:1008.
Coughlan KA, Balon TW, Valentine RJ, Petrocelli R, Schultz V, Brandon A, Cooney GJ, Kraegen EW, Ruderman NB, Saha AK. Nutrient excess and AMPK downregulation in incubated skeletal muscle and muscle of glucose infused rats. PLoS ONE. 2015;10: e0127388. https://doi.org/10.1371/journal.pone.0127388.
Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: is it a consequence of AMPK downregulation? Cell Cycle. 2011;10:3447–51. https://doi.org/10.4161/cc.10.20.17886.
Wu Y, Song P, Xu J, Zhang M, Zou MH. Withdrawal: activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2019;294:10741. https://doi.org/10.1074/jbc.W119.009747.
Valentine RJ, Coughlan KA, Ruderman NB, Saha AK. Insulin inhibits AMPK activity and phosphorylates AMPK Ser485/491 through Akt in hepatocytes, myotubes and incubated rat skeletal muscle. Arch Biochem Biophys. 2014;562:62–9. https://doi.org/10.1016/j.abb.2014.08.013.
Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, Jelenik T, Müller J, Herder C, Nowotny P, et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci USA. 2014;111:9597–602. https://doi.org/10.1073/pnas.1409229111.
Blagosklonny MV. TOR-centric view on insulin resistance and diabetic complications: perspective for endocrinologists and gerontologists. Cell Death Dis. 2013;4: e964. https://doi.org/10.1038/cddis.2013.506.
Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–36. https://doi.org/10.1038/nrendo.2014.171.
Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–78. https://doi.org/10.1152/physrev.00011.2008.
Srivastava RAK, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases: thematic review series: new lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J Lipid Res. 2012;53:2490–514. https://doi.org/10.1194/jlr.R025882.
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. https://doi.org/10.1038/nature02440.
Liang Z, Li T, Jiang S, Xu J, Di W, Yang Z, Hu W, Yang Y. AMPK: a novel target for treating hepatic fibrosis. Oncotarget. 2017;8(37):62780.
Glosse P, Föller M. AMP-activated protein kinase (AMPK)-dependent regulation of renal transport. Int J Mol Sci. 2018;19(11):3481.
Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002;22:325–46. https://doi.org/10.1146/annurev.nutr.22.010402.102912.
Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–7. https://doi.org/10.1126/science.1104343.
Munday MR, Campbell DG, Carling D, Hardie DG. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur J Biochem. 1988;175:331–8. https://doi.org/10.1111/j.1432-1033.1988.tb14201.x.
Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. https://doi.org/10.1016/s0092-8674(03)00929-2.
Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9:2439–46. https://doi.org/10.1002/j.1460-2075.1990.tb07420.x.
Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci USA. 2007;104:12017–22. https://doi.org/10.1073/pnas.0705070104.
Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 2002;99:15983–7. https://doi.org/10.1073/pnas.252625599.
Patade GR, Marita AR. Metformin: a journey from countryside to the bedside. J Obes Metab Res. 2014;1(2):127.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. https://doi.org/10.1172/JCI13505.
Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–14.
Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–65. https://doi.org/10.1016/j.cmet.2010.04.001.
Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–69. https://doi.org/10.1172/jci40671.
Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32. https://doi.org/10.1074/jbc.M202489200.
LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–81. https://doi.org/10.1152/ajpendo.00453.2005.
Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–5. https://doi.org/10.1016/j.bbrc.2003.12.120.
Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhäusl W, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–9. https://doi.org/10.2337/diabetes.53.4.1052.
Bungau S, Abdel-Daim MM, Tit DM, Ghanem E, Sato S, Maruyama-Inoue M, Yamane S, Kadonosono K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid Med Cell Longev. 2019. https://doi.org/10.1155/2019/9783429.
AlBasher G, Abdel-Daim MM, Almeer R, Ibrahim KA, Hamza RZ, Bungau S, Aleya L. Synergistic antioxidant effects of resveratrol and curcumin against fipronil-triggered oxidative damage in male albino rats. Environ Sci Pollut Res. 2020;27:6505–14. https://doi.org/10.1007/s11356-019-07344-8.
Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373:545–9. https://doi.org/10.1016/j.bbrc.2008.06.077.
Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64. https://doi.org/10.2337/db06-0006.
Bungau SG, Popa V-C. Between religion and science some aspects concerning illness and healing in antiquity. Transylv Rev. 2015;24:3–18.
Hwang JT, Kwon DY, Yoon SH. AMP-activated protein kinase: a potential target for the diseases prevention by natural occurring polyphenols. N Biotechnol. 2009;26:17–22. https://doi.org/10.1016/j.nbt.2009.03.005.
Lin YC, Hung CM, Tsai JC, Lee JC, Chen YL, Wei CW, Kao JY, Way TD. Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK). J Agric Food Chem. 2010;58:9511–7. https://doi.org/10.1021/jf1019533.
Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–8. https://doi.org/10.2337/db07-1552.
Glevitzky I, Dumitrel GA, Glevitzky M, Pasca B, Otrisal P, Bungau S, Cioca G, Pantis C, Popa M. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev Chim. 2019;70:3103–7.
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. https://doi.org/10.1038/nature05354.
Park CE, Kim MJ, Lee JH, Min BI, Bae H, Choe W, Kim SS, Ha J. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp Mol Med. 2007;39:222–9. https://doi.org/10.1038/emm.2007.25.
Kim T, Davis J, Zhang AJ, He X, Mathews ST. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun. 2009;388:377–82. https://doi.org/10.1016/j.bbrc.2009.08.018.
Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci USA. 2007;104:13632–7. https://doi.org/10.1073/pnas.0706290104.
Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130:1115–23. https://doi.org/10.1038/sj.bjp.0703397.
de la Luz C-G, Olivares-Vicente M, Herranz-López M, Arraez-Roman D, Fernández-Arroyo S, Micol V, Segura-Carretero A. Bioassay-guided purification of Lippia citriodora polyphenols with AMPK modulatory activity. J Funct Foods. 2018;46:514–20. https://doi.org/10.1016/j.jff.2018.05.026.
Olivares-Vicente M, Sánchez-Marzo N, Encinar JA, Cádiz-Gurrea MD, Lozano-Sánchez J, Segura-Carretero A, Arraez-Roman D, Riva C, Barrajón-Catalán E, Herranz-López M, Micol V. The potential synergistic modulation of AMPK by Lippia citriodora compounds as a target in metabolic disorders. Nutrients. 2019;11(12):2961. https://doi.org/10.3390/nu11122961.
Campbell NK, Fitzgerald HK, Fletcher JM, Dunne A. Plant-derived polyphenols modulate human dendritic cell metabolism and immune function via AMPK-dependent induction of heme oxygenase-1. Front Immunol. 2019;1(10):345. https://doi.org/10.3389/fimmu.2019.00345.
Tan Y, Kim J, Cheng J, Ong M, Lao WG, Jin XL, Lin YG, Xiao L, Zhu XQ, Qu XQ. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J Gastroenterol. 2017;23(21):3805. https://doi.org/10.3748/wjg.v23.i21.3805.
Vlavcheski F, Naimi M, Murphy B, Hudlicky T, Tsiani E. Rosmarinic acid, a rosemary extract polyphenol, increases skeletal muscle cell glucose uptake and activates AMPK. Molecules. 2017;22(10):1669. https://doi.org/10.3390/molecules22101669.
Xiong R, Zhou XG, Tang Y, Wu JM, Sun YS, Teng JF, Pan R, Law BY, Zhao Y, Qiu WQ, Wang XL. Lychee seed polyphenol protects the blood–brain barrier through inhibiting Aβ (25–35)-induced NLRP3 inflammasome activation via the AMPK/mTOR/ULK1-mediated autophagy in bEnd. 3 cells and APP/PS1 mice. Phytother Res. 2020. https://doi.org/10.1002/ptr.6849.
Kim H, Krenek KA, Fang C, Minamoto Y, Markel ME, Suchodolski JS, Talcott ST, Mertens-Talcott SU. Polyphenolic derivatives from mango (Mangifera Indica L.) modulate fecal microbiome, short-chain fatty acids production and the HDAC1/AMPK/LC3 axis in rats with DSS-induced colitis. J Funct Foods. 2018;48:243–51. https://doi.org/10.1016/j.jff.2018.07.011.
Wang Y, Li X, Guo Y, Chan L, Guan X. Alpha-lipoic acid increases energy expenditure by enhancing AMPK-PGC-1α signalling in the skeletal muscle of aged mice. Metabolism. 2010;59:967–76. https://doi.org/10.1016/j.metabol.2009.10.018.
Park KG, Min AK, Koh EH, Kim HS, Kim MO, Park HS, Kim YD, Yoon TS, Jang BK, Hwang JS, et al. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology. 2008;48:1477–86. https://doi.org/10.1002/hep.22496.
Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–33. https://doi.org/10.1038/nm1061.
Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, et al. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:2488–94. https://doi.org/10.1161/01.ATV.0000190667.33224.4c.
Lee Y, Naseem RH, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Alpha-lipoic acid prevents lipotoxic cardiomyopathy in acyl CoA-synthase transgenic mice. Biochem Biophys Res Commun. 2006;344:446–52. https://doi.org/10.1016/j.bbrc.2006.03.062.
Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol. 2007;293:C1395–403. https://doi.org/10.1152/ajpcell.00115.2007.
Gruzman A, Shamni O, Ben Yakir M, Sandovski D, Elgart A, Alpert E, Cohen G, Hoffman A, Katzhendler Y, Cerasi E, et al. Novel D-xylose derivatives stimulate muscle glucose uptake by activating AMP-activated protein kinase alpha. J Med Chem. 2008;51:8096–108. https://doi.org/10.1021/jm8008713.
Kim E, Kim YS, Kim KM, Jung S, Yoo SH, Kim Y. D-Xylose as a sugar complement regulates blood glucose levels by suppressing phosphoenolpyruvate carboxylase (PEPCK) in streptozotocin-nicotinamide-induced diabetic rats and by enhancing glucose uptake in vitro. Nutr Res Pract. 2016;10:11–8. https://doi.org/10.4162/nrp.2016.10.1.11.
Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48: e224. https://doi.org/10.1038/emm.2016.16.
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. https://doi.org/10.1038/nm1135.
Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–56. https://doi.org/10.1152/ajpendo.00211.2007.
Mohanan P, Subramaniyam S, Mathiyalagan R, Yang DC. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2018;42(2):123–32. https://doi.org/10.1016/j.jgr.2017.01.008.
Wang CCL, Galinkin JL, Hiatt WR. Toxicity of a novel therapeutic agent targeting mitochondrial complex I. Clin Pharmacol Ther. 2015;98:551–9. https://doi.org/10.1002/cpt.178.
Guigas B, Sakamoto K, Taleux N, Reyna SM, Musi N, Viollet B, Hue L. Beyond AICA riboside: in search of new specific AMP-activated protein kinase activators. IUBMB Life. 2009;61:18–26. https://doi.org/10.1002/iub.135.
Göransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549–60. https://doi.org/10.1074/jbc.M706536200.
Xiao B, Sanders MJ, Carmena D, Bright NJ, Haire LF, Underwood E, Patel BR, Heath RB, Walker PA, Hallen S, et al. Structural basis of AMPK regulation by small molecule activators. Nat Commun. 2013. https://doi.org/10.1038/ncomms4017.
Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–11. https://doi.org/10.1016/s1369-5266(98)80264-1.
Valgimigli M. The remarkable story of a wonder drug, which now comes to an end in the primary prevention setting: say bye-bye to aspirin! Eur Heart J. 2019;40:618–20. https://doi.org/10.1093/eurheartj/ehy872.
Jeffreys D. Aspirin: the remarkable story of a wonder drug. 1st ed. USA: Bloomsbury; 2008.
Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. https://doi.org/10.1126/science.1215327.
Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–94. https://doi.org/10.2337/dc07-1338.
Goldfine AB, Conlin PR, Halperin F, Koska J, Permana P, Schwenke D, Shoelson SE, Reaven PD. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia. 2013;56:714–23. https://doi.org/10.1007/s00125-012-2819-3.
Gómez-Galeno JE, Dang Q, Nguyen TH, Boyer SH, Grote MP, Sun Z, Chen M, Craigo WA, van Poelje PD, MacKenna DA, et al. A potent and selective AMPK activator that inhibits de novo lipogenesis. ACS Med Chem Lett. 2010;1:478–82. https://doi.org/10.1021/ml100143q.
Hunter R, Foretz M, Bultot L, Fullerton M, Deak M, Ross F, Hawley S, Shpiro N, Viollet B, Barron D, et al. Mechanism of action of compound-13: an α1-selective small molecule activator of AMPK. Chem Biol. 2014;21:866–79. https://doi.org/10.1016/j.chembiol.2014.05.014.
Mo Y, Zhu J, Jiang A, Zhao J, Ye L, Han B. Compound 13 activates AMPK-Nrf2 signaling to protect neuronal cells from oxygen glucose deprivation-reoxygenation. Aging (Albany NY). 2019;11:12032–42. https://doi.org/10.18632/aging.102534.
Zadra G, Photopoulos C, Tyekucheva S, Heidari P, Weng QP, Fedele G, Liu H, Scaglia N, Priolo C, Sicinska E, et al. A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO Mol Med. 2014;6:519–38. https://doi.org/10.1002/emmm.201302734.
Yuan X, Cai C, Chen S, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. 2014;33:2815–25. https://doi.org/10.1038/onc.2013.235.
Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–65. https://doi.org/10.1111/j.1432-1033.1995.tb20498.x.
Sullivan JE, Carey F, Carling D, Beri RK. Characterisation of 5′-AMP-activated protein kinase in human liver using specific peptide substrates and the effects of 5′-AMP analogues on enzyme activity. Biochem Biophys Res Commun. 1994;200:1551–6. https://doi.org/10.1006/bbrc.1994.1627.
Bergeron R, Previs SF, Cline GW, Perret P, Russell RR, Young LH, Shulman GI. Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–82. https://doi.org/10.2337/diabetes.50.5.1076.
Buhl ES, Jessen N, Schmitz O, Pedersen SB, Pedersen O, Holman GD, Lund S. Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner. Diabetes. 2001;50:12–7. https://doi.org/10.2337/diabetes.50.1.12.
Esquejo RM, Salatto CT, Delmore J, Albuquerque B, Reyes A, Shi Y, Moccia R, Cokorinos E, Peloquin M, Monetti M, Barricklow J. Activation of liver AMPK with PF-06409577 corrects NAFLD and lowers cholesterol in rodent and primate preclinical models. EBioMedicine. 2018;31:122–32.
Li XF, Liu XM, Huang DR, Cao HJ, Wang JY. PF-06409577 activates AMPK signalling to protect retinal pigment epithelium cells from UV radiation. Biochem Biophys Res Commun. 2018;501(1):293–9.
Saltto CT, Miller RA, Cameron KO, Cokorinos E, Reyes A, Ward J, Calbrese MF, Kurumbail RG, Rajamohan F, Kalgutkar AS, et al. Selective activation of AMPK B1-containing isoforms improves kidney function in a rat model of diabetic nephropathy. J Pharmacol Exp Ther. 2017;361(2):303–11.
Myers RW, Guan H-P, Ehrhart J, Petrov A, Prahalada S, Tozzo E, Yang X, Kurtz MM, Yang X, Trujillo M. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science. 2017;357(6350):507–11.
Schiattarella GG, Hill JA, Erhart J, Petrov A, Prahalada S, Tozzo E, Yang X, Kurtz MM, Trujillo M, Trotter DG, Feng D. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science. 2017;357(6350):507–11.
Cokorinos EC, Delmore J, Reyes AR, Albuquerque B, Kjøbsted R, Jørgensen NO, Tran JL, Jatkar A, Cialdea K, Esquejo RM, Meissen J. Activation of skeletal muscle AMPK promotes glucose disposal and glucose lowering in non-human primates and mice. Cell metab. 2017;25(5):1147–59.
Sun G, You Y, Li H, Cheng Y, Qian M, Zhou X, Yuan H, Xu QL, Dai L, Wang P, Cheng K. Discovery of AdipoRon analogues as novel AMPK activators without inhibiting mitochondrial complex I. Eur J Med Chem. 2020;200: 112466.
Schmoll D, Ziegler N, Viollet B, Foretz M, Even PC, Azzout-Marniche D, Nygaard Madsen A, Illemann M, Mandrup K, Feigh M, Czech J. Activation of adenosine monophosphate—activated protein kinase reduces the onset of diet-induced hepatocellular carcinoma in mice. Hepatol Commun. 2020;4(7):1056–72.
Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–94. https://doi.org/10.2337/diabetes.51.10.2886.
Olivier S, Foretz M, Viollet B. Promise and challenges for direct small molecule AMPK activators. Biochem Pharmacol. 2018;153:147–58. https://doi.org/10.1016/j.bcp.2018.01.049.
Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 1985;2009(106):1374–84. https://doi.org/10.1152/japplphysiol.91397.2008.
McKiernan SH, Colman RJ, Lopez M, Beasley T, Aiken JM, Anderson RM, Weindruch R. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp Gerontol. 2011;46:23–9. https://doi.org/10.1016/j.exger.2010.09.011.
Lu Q, Li X, Liu J, Sun X, Rousselle T, Ren D, Tong N, Li J. AMPK is associated with the beneficial effects of antidiabetic agents on cardiovascular diseases. Biosci Rep. 2019;39: BSR20181995. https://doi.org/10.1042/bsr20181995.
Singh M, Sharma R, Kumar A. Safety of SGLT2 inhibitors in patients with diabetes mellitus. Curr Drug Saf. 2019;14(2):87–93.
Singh M, Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf. 2018;13(2):84–91.
Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal D, Capri M, Franceschi C, Zhang Y, Becker K, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–51. https://doi.org/10.1111/acel.12088.
O’Brien AJ, Villani LA, Broadfield LA, Houde VP, Galic S, Blandino G, Kemp BE, Tsakiridis T, Muti P, Steinberg GR. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochem J. 2015;469:177–87. https://doi.org/10.1042/BJ20150122.
Ducommun S, Ford RJ, Bultot L, Deak M, Bertrand L, Kemp BE, Steinberg GR, Sakamoto K. Enhanced activation of cellular AMPK by dual-small molecule treatment: AICAR and A769662. Am J Physiol Endocrinol Metab. 2014;306:E688–96. https://doi.org/10.1152/ajpendo.00672.2013.
Bultot L, Jensen TE, Lai YC, Madsen ALB, Collodet C, Kviklyte S, Deak M, Yavari A, Foretz M, Ghaffari S, et al. Benzimidazole derivative small-molecule 991 enhances AMPK activity and glucose uptake induced by AICAR or contraction in skeletal muscle. Am J Physiol Endocrinol Metab. 2016;311:E706–19. https://doi.org/10.1152/ajpendo.00237.2016.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors have equally contributed to this paper. All authors have read and agreed with the final form of this manuscript. Conceptualization: TB and AG; software: TB and OF; investigation: TB, AG, SB, OF, and CCD; writing—original draft preparation: TB, AG, and SB; writing—review and editing: TB, SB, and CCD; supervision: TB and SBU; proof read: SB and AAH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Behl, T., Gupta, A., Sehgal, A. et al. A spotlight on underlying the mechanism of AMPK in diabetes complications. Inflamm. Res. 70, 939–957 (2021). https://doi.org/10.1007/s00011-021-01488-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01488-5