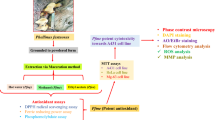
Abstract
The current study was conducted to evaluate the antiproliferative and oxidative damage protection potential of endophytic fungi Aspergillus fumigatus and Chaetomium globosum isolated from Moringa oleifera. The chloroformic extract (CE) of both the fungi showed dose dependent antiproliferative activity against human prostate adenocarcinoma (PC-3) cell line with (IC50) value of 0.055 mg/ml and 0.008 mg/ml, respectively. Further, CE of both the fungi was studied for their ability to induce apoptosis in PC-3 cell line. Various deformities in the cancerous cells treated with CE of both the fungi have been observed by confocal microscopy which indicates the cell death by apoptosis. Further apoptosis inducing ability of CE of both the fungi was observed using various flow cytometric studies. The chloroformic extract of both the fungi showed slight increase in the level of reactive oxygen species to induce apoptosis. It also showed arrest of cancerous cells at G0/G1 phase of cell cycle to induce apoptosis. The externalization of phosphatidylserine (PS) to induce apoptosis was also observed when analysed using Annexin V-FITC/PI double staining assay where the CE of A. fumigatus and C. globosum showed the total apoptosis of 94.2% and 90.3%, respectively, at the highest tested concentration of GI70. The CE of both the fungi further showed the protective behaviour for plasmid DNA pBR322, when tested for their effect against the oxidative stress caused by the Fenton’s reagent. Thus, the studies demonstrated a good antiproliferative and oxidative damage protection potential of the endophytic fungi.






Similar content being viewed by others
References
Law, J. W. F., Law, L. N. S., Letchumanan, V., Tan, L. T. H., Wong, S. H., Chan, K. G. A., Mutalib, N. S., & Lee, L. H. (2020). Anticancer drug discovery from microbial sources: The unique mangrove Streptomycetes. Molecules, 25, 5365.
Reuter, S., Gupta, S. C., Chaturvedi, M. M., & Aggarwal, B. B. (2010). Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biology and Medicine, 49, 1603–1616.
Newman, D. J., & Crag, G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products, 79, 629–661.
Petrini, O. (1991). Fungal endophyte of tree leaves. In J. H. Andrews & S. S. Hirana (Eds.), Microbial Ecology of leaves (pp. 179–197). Springer Verlag.
Amirita, A., Sindhu, P., Swetha, J., Vasanthi, N. S., & Kannan, K. P. (2012). Enumeration of endophytic fungi from medicinal plants and screening of extracellular enzymes. World Journal of Microbiology and Biotechnology, 2, 13–19.
Li, Y., Lu, C., Hu, Z., Huang, Y., & Shen, Y. (2009). Secondary metabolites of Tubercularia sp. TF5, an endophytic fungal strain of Taxus mairei. Natural Product Research, 23, 70–76.
Schulz, B., & Boyle, C. (2005). The endophytic continuum. Mycological Research, 109, 661–686.
Li, H., Qing, C., Zhang, Y., & Zhao, Z. (2005). Screening for endophytic fungi with antitumor and antifungal activities from Chinese medicinal plants. World Journal of Microbiology and Biotechnology, 27, 3005–3008.
Wiyakrutta, S., Sriubolmas, N., Panphut, W., Thongon, N., Danwisetkanjana, K., Ruangrungsi, N., & Meevootisom, V. (2004). Endophytic fungi with anti-microbial, anti-cancer and anti-malarial activities isolated from Thai medicinal plants. World Journal of Microbiology and Biotechnology, 20, 265–272.
Chuang, K. T., Wong, T. Y., Wei, Y., Huang, Y. W., & Lin, Y. (1998). Tannins and human health: A review. Critical Reviews in Food Science and Nutrition, 38, 421–464.
Fahey, J. W. (2005). Moringa oleifera: A review of the medicinal evidence for its nutritional, therapeutic and prophylactic properties. Part 1. Trees Life Journal, 47, 123–157.
Bolin, C., & andSatyabrat, G. . (2011). Antibacterial activity of the methanolic extract of stem bark of Spondias pinnata, Moringa oleifera and Alstonia scholaris. Asian Journal of Traditional Medicines, 6, 163–167.
Caceres, A., Saravia, A., Rizzo, S., Zabala, L., Leon, E. D., & Nave, F. (1992). Pharmacologic properties of Moringa oleifera 2: Screening for antispasmodic, anti-inflammatory and diuretic activity. Journal of Ethnopharmacology, 36, 233–237.
Mughal, M. H., Ali, G., Srivastava, P. S., & Iqbal, M. (1999). Improvement of drumstick [Moringa pterygosperma Gaertn.] – a unique source of food and medicine through tissue culture. Hamdard Medcus, 42, 37–42.
Goyal, B. R., Agrawal, B. B., Goyal, R. K., & Mehta, A. A. (2007). Phyto-pharmacology of Moringa oleifera Lam. ὀ. An overview. Natural Product Radiance, 6, 347–353.
Shapiro, G. I. (2006). Cyclin-dependent kinase pathways as targets for cancer treatment. Journal of Clinical Oncology, 24, 1770–1783.
Arora, D. S., & Kaur, N. (2019). Antimicrobial potential of fungal endophytes from Moringa oleifera. Applied Biochemistry and Biotechnology, 18, 628–648.
Militao, G. C. G., Danta, I. N. F., Pessoa, C., Falcão, M. J. C., Silveira, E. R., Lima, M. A. S., Curi, R., Lima, T., Moraes, M. O., & Costa-Lotufo, L. V. (2006). Induction of apoptosis by pterocarpans from Platymiscium floribundum in HL-60 human leukemia cells. Life Sciences, 78, 2409–2417.
Zheng, L., Wang, X., Luo, W., Zhan, Y., & Zhang, Y. (2013). Brucine, an effective natural compound derived from nux-vomica, induces G1 phase arrest and apoptosis in LoVo cells. Food and Chemical Toxicology, 58, 332–339.
Remesh, A. (2017). Toxicities of anticancer drugs and its management. International Journal of Basic and Clinical Pharmacology, 1, 2–12.
Nascimento do, A. M., Conti, R., Turatti, I. C., Cavalcanti, B. C., Costa-Lotufo, L. V., Pessoa, C., Moraes, M. O., Manfrim, V., Toledo, J. S., Cruz, A. K., & Pupo, M. T. (2012). Bioactive extracts and chemical constituents of two endophytic strains of Fusarium oxysporum. Rev Bras Farmacogn, 22, 1276–81.
Zhan, J., Burns, A. M., Liu, M. X., Faeth, S. H., & Gunatilaka, A. L. (2007). Search for cell motility and angiogenesis inhibitors with potential anticancer activity: Beauvericin and other constituents of two endophytic strains of Fusarium oxysporum. Journal of Natural Products, 70, 227–232.
Kaur, N., Arora, D. S., Kalia, N., & Kaur, M. (2020). Antibiofilm, antiproliferative, antioxidant and antimutagenic activities of an endophytic fungus Aspergillus fumigatus from Moringa oleifera. Molecular Biology Reports, 47, 2901–2911.
Kaur, N., Arora, D. S., Kalia, N., & Kaur, M. (2020). Bioactive potential of endophytic fungus Chaetomium globosum and GC–MS analysis of its responsible components. Scientific Reports, 10, 1–10.
Zhu, M., Zhang, X., Feng, H., Dai, J., Li, J., Che, Q., Gu, Q., Zhu, T., & Li, D. (2017). Penicisulfuranols A-F, alkaloids from the mangrove endophytic fungus Penicillium janthinellum HDN13-309. Journal of Natural Products, 80, 71–75.
Wang, Y. H., Feng, J. T., Zhang, Q., & Zhang, X. (2008). Optimization of fermentation condition for antibiotic production by Xenorhabdus nematophila with response surface methodology. Journal of Applied Microbiology, 104, 735–744.
Gerl, R., & Vaux, D. L. (2005). Apoptosis in the development and treatment of cancer. Carcinogenesis, 26, 263–270.
Pharamat, T., Palaga, T., Piapukiew, J., Whalley, A. J., & Sihanonth, P. (2013). Antimicrobial and anticancer activities of endophytic fungi from Mitragyna javanica Koord and Val. African Journal of Microbiological Research, 7, 5565–5572.
Radha, G., & Raghavan, S. C. (2017). BCL2: A promising cancer therapeutic target. BBA - Reviews on Cancer, 1868, 309–314.
Chan, W. H., & Yu, J. S. (2000). Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. Journal of Cellular Biochemistry, 78, 73–84.
Hug, H., Strand, S., Grambihler, A., Galle, J., Hack, V., Stremmel, W., Krammer, P. H., & Galle, P. R. (1997). Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. Journal of Biological Chemistry, 272, 28191–28193.
Serrano, J., Palmeira, C. M., Kuehl, D. W., & Wallace, K. B. (1999). Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1411, 201–205.
Murray, A. W. (2004). Recycling the cell cycle: Cyclins revisited. Cell, 116, 221–234.
Torres, K., & Horwitz, S. B. (1998). Mechanisms of taxol-induced cell death are concentration dependent. Cancer Research, 58, 3620–3626.
Zhao, L., Chen, Z., Wang, J., Yang, L., Zhao, Q., Wang, J., Qi, Q., Mu, R., You, Q. D., & Guo, Q. L. (2010). Synergistic effect of 5-fluorouracil and the flavanoidoroxylin A on HepG2 human hepatocellular carcinoma and on H22 transplanted mice. Cancer Chemotherapy and Pharmacology, 65, 481–489.
Yamaguchi, L. F., Vassão, D. G., Kato, M. J., & Di Mascio, P. (2005). Biflavonoids from Brazilian pine Araucaria angustifolia as potentials protective agents against DNA damage and lipoperoxidation. Phytochemistry, 66, 2238–2247.
Soumya, K., Haridas, K. R., James, J., Sameer Kumar, V. B., Edatt, L., & Sudheesh, S. (2019). Study of In vitro antioxidant and DNA damage protection activity of a novel luteolin derivative isolated from Terminalia chebula. Journal of Taibah University for Science, 13, 755–763.
Acknowledgements
The grant provided in the form of Major Research Project (MRP) sanctioned to Prof. (Dr.) Daljit Singh Arora by UGC, New Delhi is duly acknowledged, as well as the financial support to the department in the form SAP, DST Purse etc. is also appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Informed Consent
This article does not contain any studies with human participants, so the consent to participate is not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, N., Arora, D.S., Kaur, S. et al. Antiproliferative and Oxidative Damage Protection Activities of Endophytic Fungi Aspergillus fumigatus and Chaetomium globosum from Moringa oleifera Lam.. Appl Biochem Biotechnol 193, 3570–3585 (2021). https://doi.org/10.1007/s12010-021-03625-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03625-6