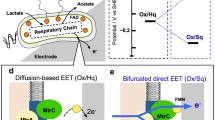
Abstract
In this study, a cell wall-associated extracellular electron transfer (EET) was determined in the thermophilic Geobacillus sp. to utilize iron as a terminal electron acceptor. The direct extracellular transfer of its electrons was primarily linked to the cell wall cytochrome-c and diffusible redox mediators like flavins during the anoxic condition. Based on the azo dye decolouration and protein film voltammetry, it was revealed that, in the absence of surface polysaccharide and diffusible mediators, the cell wall-associated EET pathway was likely to be a favorable mechanism in Geobacillus sp. Since the permeability of such redox molecule is primarily limited to the cell wall, the electron transfer occurs by direct contact with cell wall-associated cytochrome and final electron acceptor. Furthermore, transfer of electrons with the help of redox shuttling molecules like riboflavin from cytochrome to cells, vice versa indicates that Geoabcillus sp. has adopted this unique pathway during an anoxic environment for its respiration.






Similar content being viewed by others
References
Anderson LJ, Richardson DJ, Butt JN (2001) Catalytic protein film voltammetry from a respiratory nitrate reductase provides evidence for complex electrochemical modulation of enzyme activity. Biochemistry 40:11294–11307
Bae S, Lee W (2013) Biotransformation of lepidocrocite in the presence of quinones and flavins. Geochim Cosmochim Acta 114:144–215
Balasubramanian R, Levinson BT, Rosenzweig AC (2010) Secretion of flavins by three species of methanotrophic bacteria. Appl Environ Microbiol 76:7356–7358
Bartzatt R, Wol T (2014) Detection and assay of Vitamin B-2 (Riboflavin) in alkaline borate buffer with UV/Visible spectrophotometry. Int Sch Res Notices 2014:Article ID 453085
Berry EA, Trumpower BL (1987) Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem 161:1–15
Bird LJ, Bonnefoy V, Newman DK (2011) Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol 19:330–340
Brutinel ED, Gralnick JA (2012) Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Appl Microbiol Biotechnol 93:41–48
Carlson HK, Iavarone AT, Gorur A, Yeo BS, Tran R, Melnyk RA, Mathies RA, Auer M, Coates JD (2012) Surface multiheme c-type cytochromes from Thermincola potens and implications for respiratory metal reduction by Gram-positive bacteria. Proc Natl Acad Sci USA 109:1702–1707
Cole JN, Djordjevic SP, Walker MJ (2008) Isolation and solubilization of gram-positive bacterial cell wall-associated proteins. In: Posch A (ed) 2D PAGE: sample preparation and fractionation. Humana Press, Totowa, pp 295–311. https://doi.org/10.1007/978-1-60327-210-0_24
Cologgi DL, Lampa-Pastirk S, Speers AM, Kelly SD, Reguera G (2011) Extracellular reduction of uranium via Geobacter conductive pili as a protective cellular mechanism. Proc Natl Acad Sci USA 108:15248–15252
Coursolle D, Baron DB, Bond DR, Gralnick JA (2010) The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J Bacteriol 192:467–474
Dalla Vecchia E, Shao PP, Suvorova E, Chiappe D, Hamelin R, Bernier-Latmani R (2014) Characterization of the surfaceome of the metal-reducing bacterium Desulfotomaculum reducens. Front Microbiol 5:432
DE Dana JD, Gaines RV (1997) Dana’s new mineralogy: the system of mineralogy of James Dwight Dana and Edward Salisbury Dana, 8th edn. Wiley-Blackwell, Hoboken
Faustino MM, Fonseca BM, Costa NL, Lousa D, Louro RO, Paquete CM (2021) Crossing the wall: characterization of the multiheme cytochromes involved in the extracellular electron transfer pathway of Thermincola ferriacetica. Microorganisms 9:293
Fernandes AP, Nunes TC, Paquete CM, Salgueiro CA (2017) Interaction studies between periplasmic cytochromes provide insights into extracellular electron transfer pathways of Geobacter sulfurreducens. Biochem J 474:797–808
Fuller SJ, McMillan DGG, Renz MB, Schmidt M, Burke IT, Stewart DI (2014) Extracellular electron transport-mediated Fe(III) reduction by a community of alkaliphilic bacteria that use flavins as electron shuttles. Appl Environ Microbiol 80:128–137. https://doi.org/10.1128/aem.02282-13
García-Angulo VA (2017) Overlapping riboflavin supply pathways in bacteria. Crit Rev Microbiol 43:196–209
Gavrilov SN, Zavarzina DG, Elizarov IM, Tikhonova TV, Dergousova NI, Popov VO, Lloyd JR, Knight D, El-Naggar MY, Pirbadian S, Leung KM, Robb FT, Zakhartsev MV, Bretschger O, Bonch-Osmolovskaya EA (2021) Novel extracellular electron transfer channels in a gram-positive thermophilic bacterium. Front Microbiol 11:597818
Gurav R, Bhatia SK, Choi TR, Kim HJ, Song HS, Park SL, Lee SM, Lee HS, Kim SH, Yoon JJ, Yang YH (2020) Utilization of different lignocellulosic hydrolysates as carbon source for electricity generation using novel Shewanella marisflavi BBL25. J Clean Prod 277:124084
Gurumurthy DM, Neelagund SE (2010) Geobacillus sp. Iso 5, a novel amylase-producing thermophile from thermal springs in Konkan region of Southern. Indian J Earth Sci 21:319–322
Gurumurthy DM, Bharagava RN, Kumar A, Singh B, Ashfaq M, Saratale GD, Mulla SI (2019) EPS bound flavins driven mediated electron transfer in thermophilic Geobacillus sp. Microbiol Res 229:126324
Hartshorne RS et al (2007) Characterization of Shewanella oneidensis MtrC: a cell-surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors. J Biol Inorg Chem 12:1083–1094
Hernandez ME, Newman DK (2001) Extracellular electron transfer. Cell Mol Life Sci 58:1562–1571
Hess V, Poehlein A, Weghoff MC, Daniel R, Müller V (2014) A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui. BMC Genomics 15:1139
Holmes DE, Dang Y, Walker DJF, Lovley DR (2016) The electrically conductive pili of Geobacter species are a recently evolved feature for extracellular electron transfer. Microb Genom 2:000072
Kotloski NJ, Gralnick JA (2013) Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. Mbio 4:e00553-e1512
Kracke F, Vassilev I, Krömer JO (2015) Microbial electron transport and energy conservation—the foundation for optimizing bioelectrochemical systems. Front Microbiol 6:575
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature 227:680–685
Leang C, Coppi MV, Lovley DR (2003) OmcB, a c-Type Polyheme Cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol 185:2096–2103
Lloyd JR (2003) Microbial reduction of metals and radionuclides. FEMS Microbiol Rev 27:411–425
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP, Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382:445–448
Lusk BG (2019) Thermophiles; or, the Modern Prometheus: the importance of extreme microorganisms for understanding and applying extracellular electron transfer. Front Microbiol 10:818
Lusk BG, Khan QF, Parameswaran P, Hameed A, Ali N, Rittmann BE, Torres CI (2015) Characterization of Electrical current-generation capabilities from thermophilic bacterium Thermoanaerobacter pseudethanolicus using xylose, glucose, cellobiose, or acetate with fixed anode potentials. Environ Sci Technol 49:14725–14731
Lusk BG, Parameswaran P, Popat SC, Rittmann BE, Torres CI (2016) The effect of pH and buffer concentration on anode biofilms of Thermincola ferriacetica. Bioelectrochemistry 112:47–52
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci USA 105:3968–3973
Mohan SV, Velvizhi G, Krishna KV, Babu ML (2014) Microbial catalyzed electrochemical systems: a bio-factory with multi-facet applications. Bioresour Technol 165:355–364
Moody MD, Dailey HA (1983) Aerobic ferrisiderophore reductase assay and activity stain for native polyacrylamide gels. Anal Biochem 134:235–239
Mulla SI, Wang H, Sun Q, Hu A, Yu CP (2016) Characterization of triclosan metabolism in Sphingomonas sp. strain YL-JM2C. Sci Rep 6:21965
Myers CR, Myers JM (1992) Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol 174:3429–3438
Nevin KP, Lovley DR (2002) Mechanisms for Fe(III) oxide reduction in sedimentary environments. Geomicrobiol J 19:141–159
Parameswaran P, Bry T, Popat SC, Lusk BG, Rittmann BE, Torres CI (2013) Kinetic, electrochemical, and microscopic characterization of the thermophilic, anode-respiring bacterium Thermincola ferriacetica. Environ Sci Technol 47:4934–4940
Popova NA, Nikolaev YA, Tourova TP, Lysenko AM, Osipov GA, Verkhovtseva NV, Panikov NS (2002) Geobacillus uralicus, a new species of thermophilic bacteria. Microbiology 71:335–341
Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR (2005) Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101
Richter K, Schicklberger M, Gescher J (2012) Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl Environ Microbiol 78:913–921
Shi L, Belchik SM, Plymale AE, Heald S, Dohnalkova AC, Sybirna K, Bottin H, Squier TC, Zachara JM, Fredrickson JK (2011) Purification and Characterization of the [NiFe]-Hydrogenase of Shewanella oneidensis MR-1. Appl Environ Microbiol 77:5584–5590
Thomas PE, Ryan D, Levin W (1976) An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem 75:168–176
Tokunou Y, Hashimoto K, Okamoto A (2016) Acceleration of extracellular electron transfer by alternative redox-active molecules to riboflavin for outer-membrane cytochrome c of Shewanella oneidensis MR-1. J Phys Chem C 120:16168–16173
Torres CI, Marcus AK, Lee H-S, Parameswaran P, Krajmalnik-Brown R, Rittmann BE (2010) A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol Rev 34:3–17
von Canstein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623
Wall JD, Krumholz LR (2006) Uranium Reduction. Annu Rev Microbiol 60:149–166
White GF, Shi Z, Shi L, Wang Z, Dohnalkova AC, Marshall MJ, Fredrickson JK, Zachara JM, Butt JN, Richardson DJ, Clarke TA (2013) Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(III) minerals. Proc Natl Acad Sci U S A 110:6346–6351
Wu S, Xiao Y, Wang L, Zheng Y, Chang K, Zheng Z, Yang Z, Varcoe JR, Zhao F (2014) Extracellular electron transfer mediated by flavins in gram-positive Bacillus sp. WS-XY1 and Yeast Pichia stipitis. Electrochim Acta 146:564–567
Wu S, Xiao Y, Song P, Wang C, Yang Z, Slade RCT, Zhao F (2016) Riboflavin-mediated extracellular electron transfer process involving Pachysolen tannophilus. Electrochim Acta 210:117–121
Zhao F, Gurumurthy DM. 2016. Chapter 16—Resources recovery from wastewater based on extracellular electron transfer. In: Environmental materials and waste. Academic Press, pp 391–412. https://doi.org/10.1016/B978-0-12-803837-6.00016-0
Zhao J, Li F, Cao Y, Zhang X, Chen T, Song H, Wang Z (2020) Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2020.107682
Acknowledgements
We want to thank Dr. Shivayogeeswar Neelagund, Associate Professor, Department of Biochemistry, Kuvempu University, for his constant support throughout the process. DMG would like to thank all colleagues from the Department of Biotechnology, GM Institute of Technology, Davangere. SIM would like to thank all colleagues from the Department of Biochemistry, School of Applied Science, REVA University, Bangalore.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
DMG and SIM conceived and designed the study. DMG and SIM performed experimental works. DMG, LFRF, AK, GDS, MB, SKG and SIM analyzed the data. DMG, VDR and SIM wrote the paper. GDS, UG, MB, and SKG helped to revise the final draft. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors ensure no conflict of interest exist.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gurumurthy, D.M., Bilal, M., Nadda, A.K. et al. Evaluation of cell wall-associated direct extracellular electron transfer in thermophilic Geobacillus sp.. 3 Biotech 11, 383 (2021). https://doi.org/10.1007/s13205-021-02917-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02917-2