Abstract
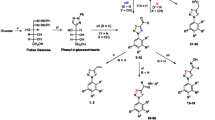
Herein, we reported the design, synthesis, antitrypanosomal and cytotoxic evaluation of a new phenoxyacetohydrazones series. All derivatives were assayed against bloodstream trypomastigote forms of T. cruzi (Y strain) and intracellular amastigotes using the model of L-929 cells infected with trypomastigotes of the Tulahuen strain. Compound (E)-N′-(3.4-dihydroxybenzylidene)-2-phenoxyacetohydrazide (11) showed activity against trypomastigotes (IC50/24 h = 10.3 µM) equivalent to that of benznidazole and with selectivity index (SI) = 46. Against infected cultures, (E)-N′-((5-nitrofuran-2-yl) methylene)-2-phenoxyacetohydrazide (19) was active at the nanomolar range (IC50/96 h = 40 nM), being about 38-fold more active than the standard drug and with SI equal to 2500. Thus, derivatives 11 and 19 could be considered a good prototypes for the development of new candidates for Chagas disease therapy.




Similar content being viewed by others
Change history
05 August 2021
The orcid id of Dr. Camila Capelini has been corrected.
References
Dumonteil E, Herrera C. Ten years of Chagas disease research: looking back to achievements, looking ahead to challenges. PLoS Negl Trop Dis. 2017;11:e0005422.
World Health Organization. https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis). Accessed 18 Jan 2021.
Conteh L, Engels T, Molyneux DH. Socioeconomic aspects of neglected tropical diseases. Lancet. 2010;375:239–247.
Antinori S, Galimberti L, Bianco R, Grande R, Galli M, Corbellino M. Chagas disease in Europe: a review for the internist in the globalized world. Eur J Intern Med. 2017;43:6–15.
Requena-Méndez A, Aldasoro E, de Lazzari E, Sicuri E, Brown M, Moore DAJ, et al. Prevalence of Chagas disease in Latin-American migrants living in Europe: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9:e0003540.
Coura JR, de Castro SL. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;97:3–24.
Patterson S, Wyllie S. Nitro drugs for the treatment of trypanosomatid diseases: past, present, and future prospects. Trends Parasitol. 2014;30:289–298.
Dias JC, Ramos AN Jr., Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR, et al. II Consenso Brasileiro em Doença de Chagas, 2015. Epidemiol Serv Saúd. 2016;25:7–86.
Thota S, Rodrigues DA, Pinheiro PSM, Lima LM, Fraga CAM, Barreiro EJ. N-Acylhydrazones as drugs. Bioorg Med Chem Lett. 2017;28:2797–2806.
Ifa D, Rodrigues CR, de Alencastro RB, Fraga CAM, Barreiro EJ. A possible molecular mechanism for the inhibition of cysteine proteases by salicylaldehyde n-acylhydrazones and related compounds. J Mol Struct 2000;505:11–17.
Serafim RAM, de Oliveira TF, Loureiro APM, Krogh R, Andricopulo AD, Dias LC, et al. Molecular modeling and structure–activity relationships studies of bioisoster hybrids of N-acylhydrazone and furoxan groups on cruzain. Med Chem Res. 2017;26:760–769.
Vergara S, Carda M, Agut R, Yepes LM, Vélez ID, Robledo SM, et al. Synthesis, antiprotozoal activity and cytotoxicity in U-937 macrophages of triclosan–hydrazone hybrids. Med Chem Res. 2017;26:3262–3273.
Ferreira RS, Dessoy MA, Pauli I, Souza ML, Krogh R, Sales AIL, et al. Biological evaluation, and structure-activity relationships of potent noncovalent and nonpeptidic cruzain inhibitors as anti-trypanosoma cruzi agents. J Med Chem. 2014;57:2380–2392.
Carvalho SA, Santa-Rita RM, de Castro SL, Fraga CAM. Synthesis and antitrypanosomal profile of new functionalized 1,3,4-thiadiazole-2-arylhydrazone derivatives, designed as non-mutagenic megazol analogues. Bioorg Med Chem Lett. 2004;14:5967–5970.
Tait A, Ganzerli S, Di Bella M. Synthesis and free radical scavenging activity of 4-(2H-1,2,4-benzothiadiazine-1, ldioxide-3-yi)-2,6-bis(1,1-dimethylethyl)phenols. Tetrah. 1996;52(38):12587–12596.
Carvalho SA, Kaiser M, Brun R, da Silva EF, Fraga CAM. Antiprotozoal activity of (E)-cinnamic N-acylhydrazone derivatives. Molecules. 2014;19(12):20374–20381.
Xu F, Wang Y, Luo D, Yu G, Wu Y, Dai A, et al. Novel trifluoromethyl pyridine derivatives bearing a 1,3,4-oxadiazole moiety as potential insecticide. Med Chem Drug Disc. 2018;3:2795–2799.
Kümmerle AE, Schmitt M, Cardozo SVS, Lugnier C, Villa P, Lopes AB, et al. Synthesis, and pharmacological evaluation of N-acylhydrazones and novel conformationally constrained compounds as selective and potent orally active phosphodiesterase-4 inhibitors. J Med Chem. 2012;55:7525–7545.
Capelini C, Câmara VRF, Villar JDF, Barbosa JMC, Salomão K, de Castro SL, et al. Synthesis, antitrypanosomal and antimycobacterial activities of coumarinic N-acylhydrazonic derivatives. Med Chem. 2020;16:1–8.
Carvalho SA. Design and synthesis of new (E)-cinnamic N-acylhydrazones as potent antitrypanosomal agents. Eur J Med Chem. 2012;54:512–521.
Glidewell C, Low JN, Wardell JL. 2-Nitrobenzaldehyde 2-Iodobenzoylhydrazone. Acta Crystallogr Sect E Struct Rep. Online. 2005;E61:o2438–o2440.
Palla G, Predieri G, Domiano P, Vignali C, Turner W. Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrah. 1986;42:3649–3654.
Romanha AJ, Castro SL, Soeiro Mde N, Lannes-Vieira J, Ribeiro I, Talvani A, et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz. 2010;105:233–238.
Raja AS, Agarwal AK, Mahajan N, Pandeya SN, Ananthan S. Antibacterial and antitubercular activities of some diphenyl hydrazones and semicarbazones. Indian J Chem B. 2010;49B:1384–1388.
Niazi S, Javali C, Paramesh M, Shivaraja S. Study of influence of linkers and substitutions on antimicrobial activity of some Schiff bases. Int J Pharm Pharm Sci. 2010;2:100–112.
Kumar PR, Yadav MS, Kumar MMK, Rao TS. Synthesis and antimicrobial activity of some new substituted aryloxy-4-thiazolidinones. J Chem. 2006;3:44–48.
Salomão K, de Souza EM, Carvalho SA, da Silva EF, Fraga CAM, Barbosa HS, et al. In vitro and in vivo activities of 1,3,4-thiadiazole-2-arylhydrazone derivatives of megazol against Trypanosoma cruzi. Antimicrob Agents Chemother. 2010;54:2023–2031.
Freitas RHCN, Barbosa JMC, Bernardino P, Sueth-Santiago V, et al. CAM. Synthesis and trypanocidal activity of novel pyridinyl-1,3,4-thiadiazole derivatives. Biomed Pharmacother. 2020;127:110162.
Jardim GAM, Reis WJ, Ribeiro MF, Ottoni FM, Alves RJ, Silva TL, et al. On the investigation of hybrid quinones: synthesis, electrochemical studies and evaluation of trypanocidal activity. RSC Adv. 2015;5:78047–78060.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The authors also thank the National Crystallographic Service, Southampton, for the X-ray data collection and the Program for Technological Development of Tools for Health-PDTIS-FIOCRUZ for use of its facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Capelini, C., de Souza, K.R., Barbosa, J.M.C. et al. Phenoxyacetohydrazones against Trypanosoma cruzi. Med Chem Res 30, 1703–1712 (2021). https://doi.org/10.1007/s00044-021-02768-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02768-9