Abstract
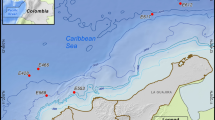
A total of 137 strains of mesophilic and psychrotolerant hydrocarbon-oxidizing bacteria have been isolated from three regions of the Sea of Japan exposed to different levels of anthropogenic pollution. The taxonomic affiliations of the culturable bacteria from surface waters and bottom sediments that are involved in the biodegradation of petroleum hydrocarbons in these regions have been identified. The isolated microorganisms belong to the phyla Actinobacteria, Firmicutes, and Proteobacteria. The hydrocarbon-oxidizing ability of the bacteria Okibacterium sp., Lechevalieria flava, Patulibacter sp., P. minatonensis, Gracilibacillus massiliensis, Thalassobacillus sp., Virgibacillus dokdonensis, Chryseomicrobium amylolyticum, Jeotgalibacillus marinus, Moraxella osloensis, Idiomarina maritima, and I. piscisalsi has been recorded for the first time. The results we obtained extend our knowledge about the potential of biodegradation of pollutants by members of certain taxa in the marine environment.


Similar content being viewed by others
REFERENCES
Alekperova, A.I., About a role of petrooxidizing bacteria in autopurification of the Samur-Absheron shelf of Caspian Sea polluted by oil, Vestn. Mosk. Gos. Obl. Univ., 2009, no. 2, pp. 6–9.
Boychenko, T.V., Khristoforova, N.K., and Buzoleva, L.S., Microbial indication of coastal waters in the northern Amur Bay, Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2009, vol. 158, pp. 324–333.
Golozubova, Yu.S., Buzoleva, L.S., Bogatyrenko, E.A., et al., Oil-oxidation properties of Micrococcus bacteria isolated from the Nakhodka Bay seawater of the Sea of Japan, Samar. Nauchn. Vestn., 2018, vol. 7, no. 2 (23), pp. 13–16.
Report on the environmental situation in Primorsky Krai in 2017, Primorsk. Gaz., 2018, no. 75 (1569).
Izrael’, Yu.A. and Tsyban’, A.V., Antropogennaya ekologiya okeana (Anthropogenic Ecology of the Ocean), Leningrad: Gidrometeoizdat, 1989.
Koronelli, T.V., Il’insky, V.V., Yanushka, V.A., and Krasnikova, T.I., Hydrocarbon-oxidizing microflora of waters of the Baltic Sea and the Curonian Lagoon polluted by a fuel oil spill, Mikrobiologiya, 1987, vol. 56, no. 3, pp. 472–478.
Koronelli, T.V., Dermicheva, S.G., and Il’insky, V.V., Species structure of hydrocarbon-oxidizing bacterial communities of aquatic ecosystems in different climatic zones, Mikrobiologiya, 1994, vol. 63, no. 5, pp. 917–923.
Ogorodnikova, A.A., Ekologo-ekonomicheskaya otsenka vozdeistviya beregovykh istochnikov zagryazneniya na prirodnuyu sredu i bioresursy zaliva Petra Velikogo (Ecological and Economic Assessment of the Impact of Coastal Sources of Pollution on the Natural Environment and Bioresources of Peter the Great Bay), Vladivostok: TINRO-Tsentr, 2001.
Buzoleva, L.S., RF Patent RU 2520084 C2, Izobret., Polezn. Modeli, 2014, no. 17.
PND F 12.1:2:2.2:2.3:3.2–03: “Metodicheskiye rekomendatsii. Otbor prob pochv, gruntov, donnykh otlozhenii, ilov, osadkov stochnykh vod, shlamov promyshlennykh stochnykh vod, otkhodov proizvodstva i potrebleniya” (Nature Conservation Federal Normative Document 12.1:2:2.2:2.3:3.2–03: Methodological Recommendations for Sampling of Soils, Grounds, Bottom Sediments, Silts, Sewage Sediments, Industrial Wastewater Sludge, Production and Consumption Waste), Moscow: Minist. Prir. Resur. Ross. Fed., 2003.
Al-Mailem, D.M., Sorkhoh, N.A., Al-Awadhi, H., et al., Biodegradation of crude oil and pure hydrocarbons by extreme halophilic archaea from hypersaline coasts of the Arabian Gulf, Extremophiles, 2010, vol. 14, pp. 321–328.
Altschul, S.F., Madden, T.L., Scháffer, A.A., et al., Gapped BLAST and PSI–BLAST: a new generation of protein database search programs, Nucleic Acids Res., 1997, vol. 25, pp. 3389–3402.
Arora, P.K., Chauhan, A., Pant, B., et al., Chryseomicrobium imtechense gen. nov., sp. nov., a new member of the family Planococcaceae, Int. J. Syst. Evol. Microbiol., 2011, vol. 61, no. 8, pp. 1859–1864.
Bogatyrenko, E.A. and Buzoleva, L.S., Characterization of the gut bacterial community of the Japanese sea cucumber Apostichopus japonicus, Microbiology (Moscow), 2016, vol. 85, no. 1, pp. 116–123.
Buzoleva, L.S., Bogatyrenko, E.A., Repina, M.A., and Belkova, N.L., Oil-oxidizing activity of bacteria isolated from south Sakhalin coastal waters, Microbiology (Moscow), 2017, vol. 86, no. 3, pp. 338–345.
Chaerun, S.K., Tazaki, K., Asada, R., and Kogure, K., Bioremediation of coastal areas 5 years after the Nakhodka oil spill in the Sea of Japan: isolation and characterization of hydrocarbon-degrading bacteria, Environ. Int., 2004, vol. 30, no. 7, pp. 911–922.
Das, N. and Chandran, P., Microbial degradation of petroleum hydrocarbon contaminants: an overview, Biotechnol. Res. Int., 2011, vol. 2011, art. ID 941810. https://doi.org/10.4061/2011/941810
Dashti, N., Ali, N., Eliyas, M., et al., Most hydrocarbonoclastic bacteria in the total environment are diazotrophic, which highlights their value in the bioremediation of hydrocarbon contaminants, Microbes Environ., 2015, vol. 30, no. 1, pp. 70–75.
Evtushenko, L.I., Dorofeeva, L.V., Krausova, V.I., et al., Okibacterium fritillariae gen. nov., sp. nov., a novel genus of the family Microbacteriaceae, Int. J. Syst. Evol. Microbiol., 2002, vol. 52, no. 3, pp. 987–993.
Farha, A.K., Tr, T., Purushothaman, A., et al., Phylogenetic diversity and biotechnological potentials of marine bacteria from continental slope of eastern Arabian Sea, J. Genet. Eng. Biotechnol., 2018, vol. 16, no. 2, pp. 253–258.
García, M.T., Gallego, V., Ventosa, A., and Mellado, E., Thalassobacillus devorans gen. nov., sp. nov., a moderately halophilic, phenol-degrading, Gram-positive bacterium, Int. J. Syst. Evol. Microbiol., 2005, vol. 55, pp. 1789–1795.
Gauze, G.F., Maksimova, T.S., Ollkhovatova, O.L., et al., Production of madumycin, an antibacterial antibiotic, by Actinomadura flava sp. nov., Antibiotiki, 1974, vol. 19, pp. 771–775.
Guan, T.-W., Tian, L., Li, E.-Y., et al., Gracilibacillus aidingensis sp. nov., a novel moderately halophilic bacterium isolated from Aiding salt lake, Arch. Microbiol., 2017, vol. 199, no. 9, pp. 1277–1281.
Hara, A., Syutsubo, K., and Harayama, S., Alcanivorax which prevails in oil-contaminated seawater exhibits broad substrate specificity for alkane degradation, Environ. Microbiol., 2003, vol. 5, no. 9, pp. 746–753.
Kumar, S., Stecher, G., and Tamura, K., MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets, Mol. Biol. Evol., 2016, vol. 33, pp. 1870–1874.
Lai, Q., Yuan, J., and Shao, Z., Altererythrobacter marinus sp. nov., isolated from deep seawater, Int. J. Syst. Evol. Microbiol., 2009, vol. 59, no. 12, pp. 2973–2976.
Lai, Q., Yu, Z., Yuan, J., et al., Nitratireductor indicus sp. nov., isolated from deep-sea water, Int. J. Syst. Evol. Microbiol., 2011, vol. 61, no. 2, pp. 295–298.
Lane, D.J., Pace, B., Olsen, G.J., et al., Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses, Proc. Natl. Acad. Sci. U. S. A., 1985, vol. 82, no. 20, pp. 6955–6959.
Takahashi, Y., Matsumoto, A., Morisaki, K., and Ōmura, S., Patulibacter minatonensis gen. nov., sp. nov., a novel actinobacterium isolated using an agar medium supplemented with superoxide dismutase, and proposal of Patulibacteraceae fam. nov., Int. J. Syst. Evol. Microbiol., 2006, vol. 56, no. 2, pp. 401–406.
Tanaka, D., Tanaka, S., Yamashiro, Y., and Nakamura, S., Distribution of oil-degrading bacteria in coastal seawater, Toyama Bay, Japan, Environ. Toxicol., 2008, vol. 23, pp. 563–569.
Wang, H.-F., Zhang, Y.-G., Li, L., et al., Okibacterium endophyticum sp. nov., a novel endophytic actinobacterium isolated from roots of Salsola affinis C. A. Mey, Antonie van Leeuwenhoek, 2015, vol. 107, no. 3, pp. 835–843.
Wang, W., Zhong, R., Shan, D., and Shao, Z., Indigenous oil-degrading bacteria in crude oil-contaminated seawater of the Yellow sea, China, Appl. Microbiol. Biotechnol., 2014, vol. 98, no. 16, pp. 7253–7269.
Zeaiter, Z., Marasco, R., Booth, J.M., et al., Phenomics and genomics reveal adaptation of Virgibacillus dokdonensis strain 21D to its origin of isolation, the seawater-brine interface of the Mediterranean Sea deep hypersaline anoxic basin discovery, Front. Microbiol., 2019, vol. 10, art. ID 1304. https://doi.org/10.3389/fmicb.2019.01304
ACKNOWLEDGMENTS
We are grateful to the research staff of the expeditions nos. 81 and 88 aboard the R/V Akademik M.A. Lavrentiev for their cooperative work, and also to the captain V.B. Ptushkin and the crew for the necessary support of our study. We also thank A.P. Tyunin, a senior researcher of the Laboratory of Biotechnology, Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far Eastern Branch, Russian Academy of Sciences, for his assistance in the molecular genetic studies.
Funding
The study was supported by the Russian Science Foundation (project no. 19-74-00028). Water and bottom sediments samples were collected during the expedition within the framework of the State assignment to the Il’ichev Pacific Oceanological Institute, Far Eastern Branch, Russian Academy of Sciences, entitled The Study of the Condition and Changes in the Natural Environment Based on Comprehensive Analysis and Modeling of Hydrometeorological, Biogeochemical, and Geological Processes and Resources of the Far East (FWMM-2019-0006), and with the financial support from the Russian Foundation for Basic Research (grants nos. 18-05-00153, 20-55-50005, and 20-35-70014), the Far East Integrated Program of Fundamental Research, Far Eastern Branch, Russian Academy of Sciences, for 2018–2020 (project nos. 20-VANT-010 and 18-1-008), and the projects from the Vietnam Academy of Science and Technology (nos. QTRU 02.02/20-21 and QTRU.02.05/19-20).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Shvetsov
Rights and permissions
About this article
Cite this article
Bogatyrenko, E.A., Kim, A.V., Dunkai, T.I. et al. Taxonomic Diversity of Culturable Hydrocarbon-Oxidizing Bacteria in the Sea of Japan. Russ J Mar Biol 47, 232–239 (2021). https://doi.org/10.1134/S1063074021030032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063074021030032