Abstract
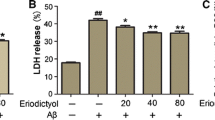
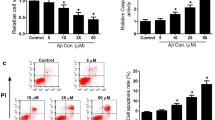
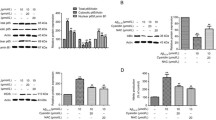
Sinensetin (SIN) is an important active compound that exists widely in citrus plants, and has been reported to exhibit various pharmacological properties, including anti-oxidative, anti-inflammatory, and anti-tumor. This study was designed to examine whether SIN can protect against amyloid beta (Aβ)-induced neurotoxicity and to elucidate the underlying mechanism. Our results showed that pretreatment with SIN for 1 h, followed by co-treatment with Aβ plus SIN for 24 h, attenuated Aβ25-35-induced cell viability reduction, oxidative stress, inflammation, and apoptosis in a dose-dependent manner. Aβ25-35-induced upregulation of Toll-like receptor 4 (TLR4) expression and nuclear translocation of nuclear factor-kappaB (NF-κB) p65 subunit were inhibited by pretreatment with SIN. Furthermore, the protective effect of SIN was abrogated by TLR4 overexpression. Hence, our data suggested that SIN attenuated Aβ25-35-induced neurotoxicity through the TLR4/NF-κB pathway.








Similar content being viewed by others
References
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344. https://doi.org/10.1056/NEJMra0909142
Tavana JP, Rosene M, Jensen NO, Ridge PG, Kauwe JS, Karch CM (2019) RAB10: an Alzheimer’s disease resilience locus and potential drug target. Clin Interv Aging 14:73–79. https://doi.org/10.2147/CIA.S159148
Guo LL, Guan ZZ, Huang Y, Wang YL, Shi JS (2013) The neurotoxicity of beta-amyloid peptide toward rat brain is associated with enhanced oxidative stress, inflammation and apoptosis, all of which can be attenuated by scutellarin. Exp Toxicol Pathol 65(5):579–584. https://doi.org/10.1016/j.etp.2012.05.003
Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai LH (2010) Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. J Neurosci 30(45):14946–14954. https://doi.org/10.1523/JNEUROSCI.4305-10.2010
Aliaga E, Silhol M, Bonneau N, Maurice T, Arancibia S, Tapia-Arancibia L (2010) Dual response of BDNF to sublethal concentrations of beta-amyloid peptides in cultured cortical neurons. Neurobiol Dis 37(1):208–217. https://doi.org/10.1016/j.nbd.2009.10.004
Huang X-F, Li J-J, Tao Y-G, Wang X-Q, Zhang R-L, Zhang J-L, Su Z-Q, Huang Q-H, Deng Y-H (2018) Geniposide attenuates Aβ25-35-induced neurotoxicity via the TLR4/NF-κB pathway in HT22 cells. RSC Adv 8(34):18926–18937. https://doi.org/10.1039/c8ra01038b
Zhou X, Yuan L, Zhao X, Hou C, Ma W, Yu H, Xiao R (2014) Genistein antagonizes inflammatory damage induced by beta-amyloid peptide in microglia through TLR4 and NF-kappaB. Nutrition 30(1):90–95. https://doi.org/10.1016/j.nut.2013.06.006
Thummayot S, Tocharus C, Jumnongprakhon P, Suksamrarn A, Tocharus J (2018) Cyanidin attenuates Abeta25-35-induced neuroinflammation by suppressing NF-kappaB activity downstream of TLR4/NOX4 in human neuroblastoma cells. Acta Pharmacol Sin 39(9):1439–1452. https://doi.org/10.1038/aps.2017.203
Perry VH (2004) The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun 18(5):407–413. https://doi.org/10.1016/j.bbi.2004.01.004
Nakanishi M, Hino M, Yoshimura M, Amakura Y, Nomoto H (2019) Identification of sinensetin and nobiletin as major antitrypanosomal factors in a citrus cultivar. Exp Parasitol 200:24–29. https://doi.org/10.1016/j.exppara.2019.03.008
Lyckander IM, Malterud KE (1996) Lipophilic flavonoids from Orthosiphon spicatus prevent oxidative inactivation of 15-lipoxygenase. Prostaglandins Leukot Essent Fatty Acids 54(4):239–246. https://doi.org/10.1016/s0952-3278(96)90054-x
Laavola M, Nieminen R, Yam MF, Sadikun A, Asmawi MZ, Basir R, Welling J, Vapaatalo H, Korhonen R, Moilanen E (2012) Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation. Planta Med 78(8):779–786. https://doi.org/10.1055/s-0031-1298458
Shin HS, Kang SI, Yoon SA, Ko HC, Kim SJ (2012) Sinensetin attenuates LPS-induced inflammation by regulating the protein level of IkappaB-alpha. Biosci Biotechnol Biochem 76(4):847–849. https://doi.org/10.1271/bbb.110908
Tan KT, Lin MX, Lin SC, Tung YT, Lin SH, Lin CC (2019) Sinensetin induces apoptosis and autophagy in the treatment of human T-cell lymphoma. Anticancer Drugs 30(5):485–494. https://doi.org/10.1097/CAD.0000000000000756
Guevara I, Iwanejko J, Dembinska-Kiec A, Pankiewicz J, Wanat A, Anna P, Golabek I, Bartus S, Malczewska-Malec M, Szczudlik A (1998) Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin Chim Acta 274(2):177–188. https://doi.org/10.1016/s0009-8981(98)00060-6
Butterfield DA, Lauderback CM (2002) Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med 32(11):1050–1060. https://doi.org/10.1016/s0891-5849(02)00794-3
Reiss AB, Arain HA, Stecker MM, Siegart NM, Kasselman LJ (2018) Amyloid toxicity in Alzheimer’s disease. Rev Neurosci 29(6):613–627. https://doi.org/10.1515/revneuro-2017-0063
Viola KL, Klein WL (2015) Amyloid beta oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol 129(2):183–206. https://doi.org/10.1007/s00401-015-1386-3
Hwang S, Lim JW, Kim H (2017) Inhibitory effect of lycopene on amyloid-beta-induced apoptosis in neuronal cells. Nutrients. https://doi.org/10.3390/nu9080883
Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F (2018) Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 14:450–464. https://doi.org/10.1016/j.redox.2017.10.014
Wang X, Wang W, Li L, Perry G, Lee HG (1842) Zhu X (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 8:1240–1247. https://doi.org/10.1016/j.bbadis.2013.10.015
Zhou WW, Lu S, Su YJ, Xue D, Yu XL, Wang SW, Zhang H, Xu PX, Xie XX, Liu RT (2014) Decreasing oxidative stress and neuroinflammation with a multifunctional peptide rescues memory deficits in mice with Alzheimer disease. Free Radic Biol Med 74:50–63. https://doi.org/10.1016/j.freeradbiomed.2014.06.013
Agostinho P, Cunha RA, Oliveira C (2010) Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des 16(25):2766–2778. https://doi.org/10.2174/138161210793176572
Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, Cuzzoni G, Venturini L, Ricevuti G, Bongiorno AI (2012) Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int J Immunopathol Pharmacol 25(2):345–353. https://doi.org/10.1177/039463201202500204
Butterfield DA, Griffin S, Munch G, Pasinetti GM (2002) Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer’s disease brain exists. J Alzheimers Dis 4(3):193–201. https://doi.org/10.3233/jad-2002-4309
Wang SW, Wang YJ, Su YJ, Zhou WW, Yang SG, Zhang R, Zhao M, Li YN, Zhang ZP, Zhan DW, Liu RT (2012) Rutin inhibits beta-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology 33(3):482–490. https://doi.org/10.1016/j.neuro.2012.03.003
Moreira PI, Zhu X, Liu Q, Honda K, Siedlak SL, Harris PL, Smith MA, Perry G (2006) Compensatory responses induced by oxidative stress in Alzheimer disease. Biol Res 39(1):7–13. https://doi.org/10.4067/s0716-97602006000100002
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1(2):120–129. https://doi.org/10.1038/35040009
Castro RE, Santos MM, Gloria PM, Ribeiro CJ, Ferreira DM, Xavier JM, Moreira R, Rodrigues CM (2010) Cell death targets and potential modulators in Alzheimer’s disease. Curr Pharm Des 16(25):2851–2864. https://doi.org/10.2174/138161210793176563
Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA (2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311(5762):847–851. https://doi.org/10.1126/science.1115035
Paudel YN, Angelopoulou E, Piperi C, Othman I, Aamir K, Shaikh MF (2020) Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s disease (AD): from risk factors to therapeutic targeting. Cells. https://doi.org/10.3390/cells9020383
Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Al-Abed Y, Davies P, Marambaud P (2012) Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J Neurochem 120(3):461–472. https://doi.org/10.1111/j.1471-4159.2011.07594.x
Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K (2007) Role of the Toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem 20(6):947–956. https://doi.org/10.1159/000110455
Li H, Lv L, Wu C, Qi J, Shi B (2020) Methyl jasmonate protects microglial cells against beta-amyloid-induced oxidative stress and inflammation via Nrf2-dependent HO-1 pathway. Neuropsychiatr Dis Treat 16:1399–1410. https://doi.org/10.2147/NDT.S241142
Gilmore TD (2006) Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25(51):6680–6684. https://doi.org/10.1038/sj.onc.1209954
Clemens JA, Stephenson DT, Smalstig EB, Dixon EP, Little SP (1997) Global ischemia activates nuclear factor-kappa B in forebrain neurons of rats. Stroke 28(5):1073–1080; discussion 1080–1071. https://doi.org/10.1161/01.str.28.5.1073
Gabriel C, Justicia C, Camins A, Planas AM (1999) Activation of nuclear factor-kappaB in the rat brain after transient focal ischemia. Brain Res Mol Brain Res 65(1):61–69. https://doi.org/10.1016/s0169-328x(98)00330-1
Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C (1997) Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc Natl Acad Sci USA 94(6):2642–2647. https://doi.org/10.1073/pnas.94.6.2642
Mattson MP (2005) NF-κB in the survival and plasticity of neurons. Neurochem Res 30(6):883–893. https://doi.org/10.1007/s11064-005-6961-x
Youn K, Yu Y, Lee J, Jeong WS, Ho CT, Jun M (2017) Polymethoxyflavones: novel beta-secretase (BACE1) inhibitors from citrus peels. Nutrients 9(9):973. https://doi.org/10.3390/nu9090973
Moussa-Pacha NM, Abdin SM, Omar HA, Alniss H, Al-Tel TH (2020) BACE1 inhibitors: current status and future directions in treating Alzheimer’s disease. Med Res Rev 40(1):339–384. https://doi.org/10.1002/med.21622
Chen CH, Zhou W, Liu S, Deng Y, Cai F, Tone M, Tone Y, Tong Y, Song W (2012) Increased NF-kappaB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int J Neuropsychopharmacol 15(1):77–90. https://doi.org/10.1017/S1461145711000149
Wang R, Chen S, Liu Y, Diao S, Xue Y, You X, Park EA, Liao FF (2015) All-trans-retinoic acid reduces BACE1 expression under inflammatory conditions via modulation of nuclear factor kappaB (NFkappaB) signaling. J Biol Chem 290(37):22532–22542. https://doi.org/10.1074/jbc.M115.662908
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhi, Z., Tang, X., Wang, Y. et al. Sinensetin Attenuates Amyloid Beta25-35-Induced Oxidative Stress, Inflammation, and Apoptosis in SH-SY5Y Cells Through the TLR4/NF-κB Signaling Pathway. Neurochem Res 46, 3012–3024 (2021). https://doi.org/10.1007/s11064-021-03406-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03406-x