Abstract
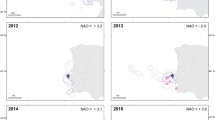
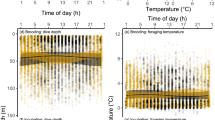
Sexual segregation, the differential space, habitat or resource use by males and females, can have profound implications for conservation, as one sex may be more vulnerable to environmental and anthropogenic stressors. The drivers of sexual segregation, such as sex differences in body size, breeding constraints, and social behaviour, have been well studied in adults but are poorly understood in immature animals. To determine whether sexual segregation occurs in juvenile Antarctic fur seals, Arctocephalus gazella, and investigate the underlying drivers, we deployed Global Location Sensors on 26 males and 19 females of 1–3 years of age at Bird Island, South Georgia. Sexual segregation occurred in foraging distribution, primarily in latitude, with females foraging closer to South Georgia and the Polar Front, and males foraging further south near the Antarctic Peninsula. This segregation was particularly evident in Feb–Apr and May–Nov, and males spent more time hauled out than females in May–Nov. Although juveniles have no immediate reproductive commitments, reproductive selection pressures are still likely to operate and drive sex differences in body size, risk-taking, and social roles. These factors, coupled with prey distribution, likely contributed to sexual segregation in juvenile Antarctic fur seals. Consequently, male and female juveniles may compete with different fisheries and respond differently to environmental change, highlighting the importance of considering sex and age groups in species conservation efforts.





Similar content being viewed by others
Data Availability
The datasets for the current study are available from the British Antarctic Survey Polar Data Centre.
References
Arnould JP, Duck CD (1997) The cost and benefits of territorial tenure, and factors affecting mating success in male Antarctic fur seals. J Zool 241:649–664
Arnould JPY, Boyd IL, Socha DG (1996) Milk consumption and growth efficiency in Antarctic fur seal (Arctocephalus gazella) pups. Can J Zool 74:254–266
Arthur B, Hindell M, Bester M, Trathan P, Jonsen I, Staniland I, Oosthuizen WC, Wege M, Lea MA (2015) Return customers: foraging site fidelity and the effect of environmental variability in wide-ranging Antarctic fur seals. PLoS ONE 10:e0120888
Atkinson A, Hill SL, Pakhomov EA, Siegel V, Reiss CS, Loeb VJ, Steinberg DK, Schmidt K, Tarling GA, Gerrish L, Sailley SF (2019) Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat Clim Change 9:142–147
Benson JF, Jorgensen SJ, O’Sullivan JB, Winkler C, White CF, Garcia-Rodriguez E, Sosa-Nishizaki O, Lowe CG (2018) Juvenile survival, competing risks, and spatial variation in mortality risk of a marine apex predator. J Appl Ecol 55:2888–2897
Bishop A, Brown C, Rehberg M, Torres L, Horning M (2018) Juvenile Steller sea lion (Eumetopias jubatus) utilization distributions in the Gulf of Alaska. Mov Ecol 6:6
Bon R, Campan R (1996) Unexplained sexual segregation in polygamous ungulates: a defense of an ontogenetic approach. Behav Process 38:131–154
Boyd IL, Croxall JP, Lunn NJ, Reid K (1995) Population demography of Antarctic fur seals: the costs of reproduction and implications for life-histories. J Anim Ecol 64:505–518
Boyd IL, McCafferty DJ, Reid K, Taylor R, Walker TR (1998) Dispersal of male and female Antarctic fur seals (Arctocephalus gazella). Can J Fish Aquat 55:845–852
Boyd IL, Staniland IJ, Martin AR (2002) Distribution of foraging by female Antarctic fur seals. Mar Ecol Prog Ser 242:285–294
Calenge C (2020) AdehabitatHR: home range estimation. https://cran.r-project.org/web/packages/adehabitatHR/index.html (Accessed date: 22-Mar-2020)
Carter MI, Russell DJ, Embling CB, Blight CJ, Thompson D, Hosegood PJ, Bennett KA (2017) Intrinsic and extrinsic factors drive ontogeny of early-life at-sea behaviour in a marine top predator. Sci Rep 7:1–14
Carter MI, McClintock BT, Embling CB, Bennett KA, Thompson D, Russell DJ (2019) From pup to predator; generalized hidden Markov models reveal rapid development of movement strategies in a naïve long-lived vertebrate. Oikos 129:630–642
CCAMLR (2015) Krill—biology, ecology and fishing https://www.ccamlr.org/en/fisheries/krill-%E2%80%93-biology-ecology-and-fishing (Accessed date 31-Mar-2020)
Chown SL, Brooks CM (2019) The state and future of Antarctic environments in a global context. Annu Rev Environ Resour 44:1–30
Clutton-Brock TH, Guinness FE, Albon S (1982) Red deer: behavior and ecology of the two sexes. University of Chicago Press, Chicago
Clutton-Brock TH, Albon SD, Guinness FE (1985) Parental investment and sex differences in juvenile mortality in birds and mammals. Nature 313:131–133
Committee on Marine Mammals (1967) Standard measurements of seals. J Mammal 48:459–462
Croft DP, Morrell LJ, Wade AS, Piyapong C, Ioannou CC, Dyer JR, Chapman BB, Wong Y, Krause J (2006) Predation risk as a driving force for sexual segregation: a cross-population comparison. Am Nat 167:867–878
Darwin C (1871) The descent of man, and selection in relation to sex. John Murray, London
Doidge DW, Croxall JP (1985) Diet and energy budget of the Antarctic fur seal, Arctocephalus gazella, at South Georgia. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 543–550
Fieberg J, Kochanny CO (2005) Quantifying home-range overlap: the importance of the utilization distribution. J Wildl Manag 69:1346–1359
Forcada J, Hoffman JI (2014) Climate change selects for heterozygosity in a declining fur seal population. Nature 511:462–465
Forcada J, Staniland IJ (2009) Antarctic fur seal Arctocephalus gazella. In: Perrin WF, Thewissen TGM, Würsig B (eds) Encyclopedia of marine mammals, 2nd edn. Academic Press, Oxford, pp 36–42
Fowler SL, Costa DP, Arnould JPY, Gales NJ, Burns JM (2007) Ontogeny of oxygen stores and physiological diving capability in Australian sea lions. Funct Ecol 21:922–935
Gentry RL, Holt JR (1982) Equipment and techniques for handling northern fur seals. NOAA technical report NMFS SSRF—United States. National Maritime Fisheries Service 758
Georgiadis N (1985) Growth patterns, sexual dimorphism and reproduction in African ruminants. Afr J Ecol 23:75–87
Government of South Georgia and the South Sandwich Islands (2020) Fisheries Overview. http://www.gov.gs/fisheries/overview/ (Accessed date: 31-Mar-2020)
Hasapes SK, Comer CE (2016) Adult white-tailed deer seasonal home range and habitat composition in Northwest Louisiana. J Southeast Assoc Fish Wildl Agencies 3:243–252
Herfindal I, Linnell JD, Odden J, Nilsen EB, Andersen R (2005) Prey density, environmental productivity and home-range size in the Eurasian lynx (Lynx lynx). J Zool 265:63–71
Hindell MA, Reisinger RR, Ropert-Coudert Y et al (2020) Tracking of marine predators to protect Southern Ocean ecosystems. Nature 580:87–92
Hückstädt LA, Piñones A, Palacios DM, McDonald BI, Dinniman MS, Hofmann EE, Burns JM, Crocker DE, Costa DP (2020) Projected shifts in the foraging habitat of crabeater seals along the Antarctic Peninsula. Nat Clim Change 10:472–477
Isaac JL (2005) Potential causes and life-history consequences of sexual size dimorphism in mammals. Mamm Rev 35:101–115
Jones KA, Wood H, Ashburner JP, Forcada J, Ratcliffe N, Votier SC, Staniland IJ (2020a) Risk exposure trade-offs in the ontogeny of sexual segregation in Antarctic fur seal pups. Behav Ecol 31:719–730
Jones KA, Ratcliffe N, Votier SC, Newton J, Forcada J, Dickens J, Stowasser G, Staniland IJ (2020b) Intra-specific niche partitioning in Antarctic fur seals Arctocephalus Gazella. Sci Rep 10:3238
Joo R, Boone ME, Clay TA, Patrick SC, Clusella-Trullas S, Basille M (2020) Navigating through the R packages for movement. J Anim Ecol 89:248–267
Kenyon KW, Wilke F (1953) Migration of the northern fur seal Callorhinus ursinus. J Mammal 34:86–98
Krüger O, Wolf JB, Jonker RM, Hoffman JI, Trillmich F (2014) Disentangling the contribution of sexual selection and ecology to the evolution of size dimorphism in pinnipeds. Evolution 68:1485–1496
Le Boeuf BJ, Crocker DE, Costa DP, Blackwell SB, Webb PM, Houser DS (2000) Foraging ecology of northern elephant seals. Ecol Monogr 70:353–382
Leung ES, Chilvers BL, Nakagawa S, Moore AB, Robertson BC (2012) Sexual segregation in juvenile New Zealand sea lion foraging ranges: implications for intraspecific competition, population dynamics and conservation. PLoS ONE 7:e45389
Lindenfors P, Tullberg BS, Biuw M (2002) Phylogenetic analyses of sexual selection and sexual size dimorphism in pinnipeds. Behav Ecol Sociobiol 52:188–193
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Lisovski S, Hewson CM, Klaassen RH, Korner-Nievergelt F, Kristensen MW, Hahn S (2012) Geolocation by light: accuracy and precision affected by environmental factors. Methods Ecol Evol 3:603–612
Lisovski S, Wotherspoon S, Summer M (2016) TwGeos: basic data processing for light-level geolocation archival tags. https://rdrr.io/github/slisovski/TwGeos/ (Accessed date: 14-Mar-2020)
Lisovski S, Bauer S, Briedis M, Davidson SC, Dhanjal-Adams KL, Hallworth MT, Karagicheva J, Meier CM, Merkel B, Ouwehand J, Pedersen L, Rakhimberdiev E, Roberto-Charron A, Seavy NE, Sumner MD, Taylor CM, Wotherspoon SJ, Bridge ES (2019) Light-level geolocator analyses: a user’s guide. J Anim Ecol 89:221–236
Liwanag HEM, Williams TM, Costa DP, Kanatous SB, Davis RW, Boyd IL (2009) The effects of water temperature on the energetic costs of juvenile and adult California sea lions (Zalophus californianus): the importance of skeletal muscle thermogenesis for thermal balance. J Exp Biol 212:3977–3984
Lowther AD, Staniland I, Lydersen C, Kovacs KM (2020) Male Antarctic fur seals: neglected food competitors of bioindicator species in the context of an increasing Antarctic krill fishery. Sci Rep 10:1–12
Luque SP (2007) Diving behaviour analysis in R. R News 7:8–14
Main MB, Weckerly FW, Bleich VC (1996) Sexual segregation in ungulates: new directions for research. J Mammal 77:449–461
Mueller B, Pörschmann U, Wolf JB, Trillmich F (2011) Growth under uncertainty: the influence of marine variability on early development of Galapagos sea lions. Mar Mamm Sci 27:350–365
Murphy EJ, Thorpe SE, Watkins JL, Hewitt R (2004) Modeling the krill transport pathways in the Scotia Sea: spatial and environmental connections generating the seasonal distribution of krill. Deep Sea Res Part II 51:1435–1456
Navarro J, Votier SC, Aguzzi J, Chiesa JJ, Forero MG, Phillips RA (2013) Ecological segregation in space, time and trophic niche of sympatric planktivorous petrels. PLoS ONE 8:e62897
Nicol S, Foster J, Kawaguchi S (2012) The fishery for Antarctic krill—recent developments. Fish Fish 13:30–40
Payne MR (1979) Growth in the Antarctic fur seal Arctocephalus gazella. J Zool 187:1–20
Pellegrini AD (2004) Sexual segregation in childhood: a review of evidence for two hypotheses. Anim Behav 68:435–443
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2020) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–150, https://CRAN.R-project.org/package=nlme (Accessed date: 14-Mar-2020)
Ponganis PJ, Ponganis EP, Ponganis KV, Kooyman GL, Gentry RL, Trillmich F (1990) Swimming velocities in otariids. Can J Zool 68:2105–2112
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for statistical computing. https://www.R-project.org/ (Accessed date: 14-Mar-2020)
Reeve JP, Fairbairn DJ (2001) Predicting the evolution of sexual size dimorphism. J Evol Biol 14:244–254
Reid K (1995) The diet of Antarctic fur seals (Arctocephalus gazella Peters 1875) during winter at South Georgia. Antarct Sci 7:241–249
Reid K, Arnould JP (1996) The diet of Antarctic fur seals Arctocephalus gazella during the breeding season at South Georgia. Polar Biol 16:105–114
Riotte-Lambert L, Weimerskirch H (2013) Do naive juvenile seabirds forage differently from adults? Proc Royal Soc B 280:20131434
Rodrigues MA (2014) Emergence of sex-segregated behavior and association patterns in juvenile spider monkeys. Neotrop Primates 21:183–188
Ruckstuhl KE, Neuhaus P (2005) Sexual segregation in vertebrates: ecology of the two sexes. Cambridge University Press, Cambridge
Sæther BE, Coulson T, Grøtan V, Engen S, Altwegg R, Armitage KB, Barbraud C, Becker PH, Blumstein DT, Dobson FS, Festa-Bianchet M (2013) How life history influences population dynamics in fluctuating environments. Am Nat 182:743–759
Salton M, Kirkwood R, Slip D, Harcourt R (2019) Mechanisms for sex-based segregation in foraging behaviour by a polygynous marine carnivore. Mar Ecol Prog Ser 624:213–226
Schmidt-Nielsen K, Knut SN (1984) Scaling: why is animal size so important? Cambridge University Press, Cambridge
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–38
Schulte-Hostedde AI, Millar JS, Hickling GJ (2001) Sexual size dimorphism in body composition of small mammals. Can J Zool 79:1016–1020
Staniland IJ (2005) Sexual segregation in seals. In: Ruckstuhl KE, Clutton-Brock TH (eds) Sexual segregation in vertebrates: ecology of the two sexes. Cambridge University Press, Cambridge, pp 53–73
Staniland IJ, Robinson SL (2008) Segregation between the sexes: Antarctic fur seals, Arctocephalus gazella, foraging at South Georgia. Anim Behav 75:1581–1590
Staniland IJ, Robinson SL, Silk JRD, Warren N, Trathan PN (2012) Winter distribution and haul-out behaviour of female Antarctic fur seals from South Georgia. Mar Biol 159:291–301
Staniland IJ, Ratcliffe N, Trathan PN, Forcada J (2018) Long term movements and activity patterns of an Antarctic marine apex predator: The leopard seal. PLoS ONE 13:e0197767
Stephens DW, Krebs JR (1986) Foraging theory, vol 1. Princeton University Press, Princeton
Stokke S, Toit JT (2000) Sex and size related differences in the dry season feeding patterns of elephants in Chobe National Park, Botswana. Ecography 23:70–80
Sullivan KA (1989) Predation and starvation: age-specific mortality in juvenile juncos (Junco phaenotus). J Anim Ecol 58:275–286
Trivers RL (1972) Parental investment and sexual selection. In: Trivers RL (ed) Sexual selection and the descent of man. Aldine Publishing Company, Chicago, pp 136–179
Votier SC, Fayet AL, Bearhop S, Bodey TW, Clark BL, Grecian J, Guilford T, Hamer KC, Jeglinski JWE, Morgan G, Wakefield E, Patrick SC (2017) Effects of age and reproductive status on individual foraging site fidelity in a long-lived marine predator. Proc Royal Soc B Biol Sci 284:20171068
Waluda CM, Gregory S, Dunn MJ (2010) Long-term variability in the abundance of Antarctic fur seals Arctocephalus gazella at Signy Island South Orkneys. Polar Biol 33:305–312
Warren NL, Trathan PN, Forcada J, Fleming A, Jessopp MJ (2006) Distribution of post-weaning Antarctic fur seal Arctocephalus gazella pups at South Georgia. Polar Biol 29:179–188
Wearmouth VJ, Sims DW (2008) Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Adv Mar Biol 54:107–170
Weckerly FW (1998) Sexual-size dimorphism: influence of mass and mating systems in the most dimorphic mammals. J Mammal 79:33–52
Wood SN (2017) Mgcv. Mixed GAM computation vehicle with GCV/AIC/REML smoothness estimation. At https://stat.ethz.ch/R-manual/R-devel/library/mgcv/html/mgcv-package.html (Accessed date: 14-Mar-2020)
Wotherspoon SJ, Sumner MD, Lisovski S (2019) Solar/satellite geolocation for animal tracking. https://rdrr.io/github/SWotherspoon/SGAT/ (Accessed date: 14-Mar-2020)
Zeppelin T, Pelland N, Sterling J, Brost B, Melin S, Johnson D, Lea MA, Ream R (2019) Migratory strategies of juvenile northern fur seals (Callorhinus ursinus): bridging the gap between pups and adults. Sci Rep 9:1–16
Acknowledgements
We sincerely thank all of the British Antarctic Survey Scientists and Zoological Field Assistants that contributed to this study by deploying and retrieving GLS loggers from juvenile Antarctic fur seals at Bird Island, South Georgia. We would like to acknowledge the use of the University of Exeter's Advanced Research Computing facilities, and we thank Dr Manmohan Sharma for his assistance with using the linux server for the original version of this manuscript. Additional thanks to Dr Kimberley Bennett and Dr Robbie McDonald for their valuable feedback on the original version of this manuscript for K Jones’ PhD thesis.
Funding
This study was supported by the Natural Environment Research Council Capability Fund and the Natural Environment Research Council Great Western Four + Doctoral Training Partnership (NE/L002434/1).
Author information
Authors and Affiliations
Contributions
IJS conceived the study. All co-authors advised on analyses. IJS, NR and SL provided extracts of code to process GLS data, and ASBL provided code to calculate the Utilisation Distribution Overlap Index. KAJ analysed the data and wrote the manuscript. All co-authors edited and provided feedback on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Mathew Samuel Crowther.
We found that reproductive selection pressures are likely to operate in juveniles, driving sex differences in body size, risk-taking, and social roles, and leading to sexual segregation.
Rights and permissions
About this article
Cite this article
Jones, K.A., Ratcliffe, N., Votier, S.C. et al. Sexual segregation in juvenile Antarctic fur seals. Oecologia 197, 339–352 (2021). https://doi.org/10.1007/s00442-021-04983-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-021-04983-y