Abstract
Introduction
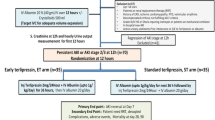
Spontaneous bacterial peritonitis (SBP) is a common infection in patients with hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACLF). SBP significantly increases the mortality rate and medical costs. The association between proton pump inhibitor (PPI) use and SBP remains unclear. We conducted a retrospective study to investigate the association between PPI use and SBP in patients with HBV-related ACLF and to explore the risk factors for SBP.
Methods
We compared the SBP incidence between the PPI and non-PPI groups before and after propensity score matching and explored the association between the duration and type of PPI and SBP occurrence. Risk factors for SBP occurrence were determined by univariate and multivariate logistic regression analysis.
Results
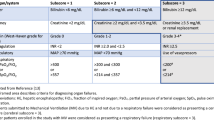
The SBP incidence was higher in the PPI group than in the non-PPI group before and after propensity score matching. The SBP incidence increased for elevated MELD scores in PPI users. There was a similar SBP incidence in both different types and durations of PPI users. MELD score, old age, male sex, and high WBC count were significant independent risk factors for SBP in PPI users with HBV-related ACLF in the hospital.
Conclusions
PPI therapy increases the risk of SBP development in patients with HBV-related ACLF. MELD score, old age, male sex, and high WBC count could serve as predictors of SBP in PPI users. Caution should be taken regarding PPI use, especially for patients with MELD scores > 30.







Similar content being viewed by others
References
Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update [published correction appears in Hepatol Int. 2019 Nov, 13(6), pp. 826–828]. Hepatol Int. 2019;13:353–90.
Shalimar, Rout G, Jadaun SS, et al. Prevalence, predictors and impact of bacterial infection in acute on chronic liver failure patients. Dig Liver Dis. 2018; 50: 1225–1231.
Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437.e14379.
Qin G, Shao JG, Zhu YC, et al. Population-representative incidence of acute-on-chronic liver failure: a prospective cross-sectional study. J Clin Gastroenterol. 2016;50:670–5.
Abbas Z, Shazi L. Pattern and profile of chronic liver disease in acute on chronic liver failure. Hepatol Int. 2015;9:366–72.
Yuan L, Zeng BM, Liu LL, et al. Risk factors for progression to acute-on-chronic liver failure during severe acute exacerbation of chronic hepatitis B virus infection. World J Gastroenterol. 2019;25:2327–37.
Fernández J, Prado V, Trebicka J, et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398–411.
Olson JC. Acute-on-chronic and decompensated chronic liver failure: definitions, epidemiology, and prognostication. Crit Care Clin. 2016;32:301–9.
Sundaram V, Jalan R, Wu T, et al. Factors associated with survival of patients with severe acute-on-chronic liver failure before and after liver transplantation. Gastroenterology. 2019; 156: 1381–1391. e3.
Zhang Y, Nie Y, Liu L, Zhu X. Assessing the prognostic scores for the prediction of the mortality of patients with acute-on-chronic liver failure: a retrospective study. PeerJ. 2020; 8: e9857.
Chen T, Yang Z, Choudhury AK, et al. Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: a multi-national study from the Asia-Pacific region. Hepatol Int. 2019;13:695–705.
Zhai XR, Tong JJ, Wang HM, et al. Infection deteriorating hepatitis B virus related acute-on-chronic liver failure: a retrospective cohort study. BMC Gastroenterol. 2020;20:320.
Fernández J, Acevedo J, Wiest R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870–80.
Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis–bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41:1116–31.
Engelmann C, Berg T. Management of infectious complications associated with acute-on-chronic liver failure. Visc Med. 2018;34:261–8.
Schwabl P, Bucsics T, Soucek K, et al. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int. 2015;35:2121–8.
Min YW, Lim KS, Min BH, et al. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther. 2014;40:695–704.
Choi EJ, Lee HJ, Kim KO, et al. Association between acid suppressive therapy and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Scand J Gastroenterol. 2011;46:616–20.
Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol. 2009;104:1130–4.
Terg R, Casciato P, Garbe C, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol. 2015;62:1056–60.
Goel GA, Deshpande A, Lopez R, Hall GS, van Duin D, Carey WD. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol. 2012;10:422–7.
Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265–72.
Mandorfer M, Bota S, Schwabl P, et al. Proton pump inhibitor intake neither predisposes to spontaneous bacterial peritonitis or other infections nor increases mortality in patients with cirrhosis and ascites. PLoS One. 2014; 9: e110503.
Pang SH, Graham DY. A clinical guide to using intravenous proton-pump inhibitors in reflux and peptic ulcers. Therap Adv Gastroenterol. 2010;3(1):11–22.
Corleto VD, Festa S, Di Giulio E, Annibale B. Proton pump inhibitor therapy and potential long-term harm. Curr Opin Endocrinol Diabetes Obes. 2014;21:3–8.
Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther. 2013;37:1132–56.
O’Leary JG, Bajaj JS, Tandon P, et al. Outcomes after listing for liver transplant in patients with acute-on-chronic liver failure: the multicenter North American consortium for the study of end-stage liver disease experience. Liver Transpl. 2019;25:571–9.
European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397–417.
Nakayama N, Uemura H, Uchida Y, et al. A multicenter pilot survey to clarify the clinical features of patients with acute-on-chronic liver failure in Japan. Hepatol Res. 2018;48:303–12.
Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure [published correction appears in Hepatology. 2011 Sep 2;54(3):1114]. Hepatology. 2011;53(3):774–780.
Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Shi Y, Zheng M. Hepatitis B virus persistence and reactivation. BMJ. 2020;370:m2200.
Shi Y, Yang Y, Hu Y, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62(1):232–42.
Kabbani AR, Schultalbers M, Tergast T, et al. Influence of a spontaneous bacterial peritonitis, nosocomial infections and acute-on-chronic liver failure on treatment revenues in patients with decompensated cirrhosis in Germany. Z Gastroenterol. 2020;58:855–67.
Allen AM, Kim WR, Moriarty JP, Shah ND, Larson JJ, Kamath PS. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology. 2016;64:2165–72.
Janka T, Tornai T, Borbély B, et al. Deleterious effect of proton pump inhibitors on the disease course of cirrhosis. Eur J Gastroenterol Hepatol. 2020;32:257–64.
Kwon JH, Koh SJ, Kim W, et al. Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2014;29:775–81.
Deshpande A, Pasupuleti V, Thota P, et al. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol. 2013;28:235–42.
Trikudanathan G, Israel J, Cappa J, O’Sullivan DM. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract. 2011;65:674–8.
O’Leary JG, Reddy KR, Wong F, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:753-9.e92.
Campbell MS, Obstein K, Reddy KR, Yang YX. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Dig Dis Sci. 2008;53:394–8.
Kim JH, Lim KS, Min YW, et al. Proton pump inhibitors do not increase the risk for recurrent spontaneous bacterial peritonitis in patients with cirrhosis. J Gastroenterol Hepatol. 2017;32:1064–70.
de Vos M, De Vroey B, Garcia BG, et al. Role of proton pump inhibitors in the occurrence and the prognosis of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Liver Int. 2013;33:1316–23.
Hung TH, Tseng CW, Lee HF, Tsai CC, Tsai CC. Effect of proton pump inhibitors on mortality in patients with cirrhosis and spontaneous bacterial peritonitis. Ann Hepatol. 2018;17:933–9.
Chang SS, Lai CC, Lee MG, et al. Risk of spontaneous bacterial peritonitis associated with gastric Acid suppression. Medicine (Baltimore). 2015; 94: e944.
Huang KW, Kuan YC, Luo JC, Lin CL, Liang JA, Kao CH. Impact of long-term gastric acid suppression on spontaneous bacterial peritonitis in patients with advanced decompensated liver cirrhosis. Eur J Intern Med. 2016;32:91–5.
Lutz P, Goeser F, Kaczmarek DJ, et al. Relative ascites polymorphonuclear cell count indicates bacterascites and risk of spontaneous bacterial peritonitis. Dig Dis Sci. 2017;62:2558–68.
Bajaj JS, O’Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56(6):2328–35.
Obstein KL, Campbell MS, Reddy KR, Yang YX. Association between model for end-stage liver disease and spontaneous bacterial peritonitis. Am J Gastroenterol. 2007;102:2732–6.
Yip TC, Chan HL, Tse YK, et al. On-treatment improvement of MELD score reduces death and hepatic events in patients with hepatitis B-related cirrhosis. Am J Gastroenterol. 2018;113:1629–38.
Kraja B, Sina M, Mone I, et al. Predictive Value of the Model of End-Stage Liver Disease in Cirrhotic Patients with and without Spontaneous Bacterial Peritonitis. Gastroenterol Res Pract. 2012; 2012: 539059.
Wang X, Wang BM, Jiang K, et al. The predictive value of end-stage liver disease model for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Zhonghua Nei Ke Za Zhi. 2009;48:629–32.
Khan R, Ravi S, Chirapongsathorn S, et al. Model for end-stage liver disease score predicts development of first episode of spontaneous bacterial peritonitis in patients with cirrhosis. Mayo Clin Proc. 2019;94:1799–806.
De Roza MA, Kai L, Kam JW, et al. Proton pump inhibitor use increases mortality and hepatic decompensation in liver cirrhosis. World J Gastroenterol. 2019;25:4933–44.
Yamamoto K, Ishigami M, Honda T, et al. Influence of proton pump inhibitors on microbiota in chronic liver disease patients. Hepatol Int. 2019;13:234–44.
Bajaj JS, Acharya C, Fagan A, et al. Proton pump inhibitor initiation and withdrawal affects gut microbiota and readmission risk in cirrhosis. Am J Gastroenterol. 2018;113:1177–86.
Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73.
Geddes K, Rubino SJ, Magalhaes JG, et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med. 2011;17:837–44.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Medical Writing Assistance
American Journal Experts (AJE) provided medical writing assistance for this paper; this was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Junying Qi and Tao Chen contributed to the study concept and design and contributed to the study supervision and critical revision of the manuscript for important content. Meng zhang contributed as first author and was involved in the analysis and interpretation of data, statistical analysis, and drafting of the manuscript. Meng Zhang, Xin Xu, Wei Liu, Zhongwei Zhang, Qiuyu Cheng, Zhongyuan Yang, Tingting Liu, and Yunhui Liu enrolled patients, collected clinical data, and assisted in the analysis and interpretation of clinical data. Qin Ning contributed to data re-evaluation and presentation, and manuscript edition.
Disclosures
All authors (Meng Zhang, Xin Xu, Wei Liu, Zhongwei Zhang, Qiuyu Cheng, Zhongyuan Yang, Tingting Liu, Yunhui Liu, Qin Ning, Tao Chen, and Junying Qi) have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Human Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (2017-S312-2). Written informed consents were obtained from the patients.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, M., Xu, X., Liu, W. et al. Proton Pump Inhibitor Therapy Increases the Risk of Spontaneous Bacterial Peritonitis in Patients with HBV-Related Acute-on-Chronic Liver Failure. Adv Ther 38, 4675–4694 (2021). https://doi.org/10.1007/s12325-021-01844-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01844-1