Abstract
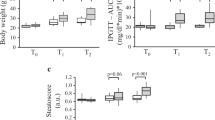
Drug-induced long QT syndrome (DI-LQTS) is fatal and known to have a higher incidence in women rather than in men. Multiple risk factors potentiate the incidence of DI-LQTS, but the actual contribution of obesity remains largely unexplored. Correspondingly, the present study is aimed to evaluate the susceptibility of DI-LQTS in WNIN/Ob rat in comparison with its lean counterpart using 3-lead electrocardiography. Four- and eight-month-old female WNIN/Ob and their lean controls were used for the experimentation. Non-invasive blood pressure measurement and total body electric conductivity (TOBEC) analysis were carried out. After the baseline evaluations, animals were anesthetized with Ketamine (50 mg/kg). Haloperidol (12.5 mg/kg single dose) was administered intraperitoneally and ECG was taken at 0, 10, 20, 30, 60 min, and 24 h time points. Myocardial lystes were used to assess the BNP, protein carbonylation, and hydroxyproline content. Adiposity, as assessed by TOBEC, is higher in obese rats with elevated mean arterial blood pressure. Baseline-corrected QT interval (QTc) is significantly higher in the obese rat with a wider QRS complex. The incidence of PVC and VT are more intense in the obese rat. Haloperidol-induced QT prolongation in obese rats was rapidly induced than in lean, which was observed to remain till 24 h in obese groups while normalized in lean controls. Higher levels of BNP, protein carbonylation, hydroxyproline content, and relative heart weights indicated the presence of cardiac hypertrophy. The study provides preliminary evidence that obesity can be a potential risk factor for DI-LQTS with faster onset and longer subsistence.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wheelan, K., Mukharji, J., Rude, R. E., Poole, W. K., Gustafson, N., Thomas, L. J., Strauss, H. W., Jaffe, A. S., Muller, J. E., Roberts, R., Croft, C. H., Passamani, E. R., & Willerson, J. T. (1986). Sudden death and its relation to QT-interval prolongation after acute myocardial infarction: Two-year follow-up. American Journal of Cardiology, 57(10), 745–750. https://doi.org/10.1016/0002-9149(86)90606-5
Nachimuthu, S., Assar, M. D., & Schussler, J. M. (2012). Drug-induced QT interval prolongation: Mechanisms and clinical management. Therapeutic Advances in Drug Safety, 3(5), 241–253. https://doi.org/10.1177/2042098612454283
Li, M., & Ramos, L. G. (2017). Drug-induced QT prolongation and torsades de pointes. Pharmacy and Therapeutics, 42(7), 473–477.
Molokhia, M., Pathak, A., Lapeyre-Mestre, M., Caturla, L., Montastruc, J. L., McKeigue, P., & L’AssociationFrançaise des CentresRégionaux de Pharmacovigilance (CRPV). (2008). Case ascertainment and estimated incidence of drug-induced long-QT syndrome: Study in Southwest France. British Journal of Clinical Pharmacology, 66(3), 386–395. https://doi.org/10.1111/j.1365-2125.2008.03229.x
Sarganas, G., Garbe, E., Klimpel, A., Hering, R. C., Bronder, E., & Haverkamp, W. (2014). Epidemiology of symptomatic drug-induced long QT syndrome and Torsade de Pointes in Germany. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 16(1), 101–108. https://doi.org/10.1093/europace/eut214
Ray, W. A., Murray, K. T., Hall, K., Arbogast, P. G., & Stein, C. M. (2012). Azithromycin and the risk of cardiovascular death. New England Journal of Medicine, 366(20), 1881–1890. https://doi.org/10.1056/NEJMoa1003833
Ray, W. A., Murray, K. T., Meredith, S., Narasimhulu, S. S., Hall, K., & Stein, C. M. (2004). Oral erythromycin and the risk of sudden death from cardiac causes. The New England Journal of Medicine, 351(11), 1089–1096. https://doi.org/10.1056/NEJMoa040582
Ray, W. A., Chung, C. P., Murray, K. T., Cooper, W. O., Hall, K., & Stein, C. M. (2015). Out-of-hospital mortality among patients receiving methadone for noncancer pain. JAMA Internal Medicine, 175(3), 420–427. https://doi.org/10.1001/jamainternmed.2014.6294
Leonard, C. E., Freeman, C. P., Newcomb, C. W., Bilker, W. B., Kimmel, S. E., Strom, B. L., & Hennessy, S. (2013). Antipsychotics and the risks of sudden cardiac death and all-cause death: Cohort studies in medicaid and dually-eligible medicaid–medicare beneficiaries of five states. Journal of Clinical & Experimental Cardiology, 10(6), 1–9. https://doi.org/10.4172/2155-9880.S10-006
Holmgren, C. M., Abdon, N. J., Bergfeldt, L. B., Edvardsson, N. G., Herlitz, J. D., Karlsson, T., & Åstrand, B. H. (2014). Changes in medication preceding out-of-hospital cardiac arrest where resuscitation was attempted. Journal of Cardiovascular Pharmacology, 63(6), 497–503. https://doi.org/10.1097/FJC.0000000000000073
Jackman, W. M., Friday, K. J., Anderson, J. L., Aliot, E. M., Clark, M., & Lazzara, R. (1988). The long QT syndromes: A critical review, new clinical observations and a unifying hypothesis. Progress in Cardiovascular Diseases, 31(2), 115–172. https://doi.org/10.1016/0033-0620(88)90014-x
Makkar, R. R., Fromm, B. S., Steinman, R. T., Meissner, M. D., & Lehmann, M. H. (1993). Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA, 270(21), 2590–2597. https://doi.org/10.1001/jama.1993.03510210076031
Drew, B. J., Ackerman, M. J., Marjorie, F., Brian, G. W., Paul, K., Venu, M., Wojciech, Z., American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, & Council on Cardiovascular Nursing. (2010). Prevention of Torsade de Pointes in hospital settings. Circulation, 121(8), 1047–1060. https://doi.org/10.1161/CIRCULATIONAHA.109.192704
Alpert, M. A., Omran, J., & Bostick, B. P. (2016). Effects of obesity on cardiovascular hemodynamics, cardiac morphology, and ventricular function. Current Obesity Reports, 5(4), 424–434. https://doi.org/10.1007/s13679-016-0235-6
Santos, C., & Marques da Silva, P. (2018). Hemodynamic patterns in obesity associated hypertension. BMC Obesity. https://doi.org/10.1186/s40608-018-0190-8
Grippo, A. J., Santos, C. M., Johnson, R. F., Beltz, T. G., Martins, J. B., Felder, R. B., & Johnson, A. K. (2004). Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. American Journal of Physiology, Heart and Circulatory Physiology, 286(2), 619–626. https://doi.org/10.1152/ajpheart.00450.2003
Watanabe, H., Tanabe, N., Watanabe, T., Darbar, D., Roden, D. M., Sasaki, S., & Aizawa, Y. (2008). Metabolic syndrome and risk of development of atrial fibrillation. Circulation, 117(10), 1255–1260. https://doi.org/10.1161/CIRCULATIONAHA.107.744466
Rossi, S., Baruffi, S., Bertuzzi, A., Mastorci, F., Sgoifo, A., Musso, E., Corradi, D., Maestri, R., Brisinda, D., Fenici, R., & Macchi, E. (2007). Susceptibility to ventricular arrhythmias in aged hearts. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, 2007, 410–414. https://doi.org/10.1109/IEMBS.2007.4352311
Kalashikam, R. R., Inagadapa, P. J. N., Thomas, A. E., Jeyapal, S., Giridharan, N. V., & Raghunath, M. (2014). Leptin gene promoter DNA methylation in WNIN obese mutant rats. Lipids in Health and Disease, 13, 25. https://doi.org/10.1186/1476-511X-13-25
Sinha, J. K., Ghosh, S., Swain, U., Giridharan, N. V., & Raghunath, M. (2014). Increased macromolecular damage due to oxidative stress in the neocortex and hippocampus of WNIN/Ob, a novel rat model of premature aging. Neuroscience, 269, 256–264. https://doi.org/10.1016/j.neuroscience.2014.03.040
Reddy, P. Y., Giridharan, N. V., Balakrishna, N., Validandi, V., Pullakhandam, R., & Reddy, G. B. (2013). Increased risk of cataract development in WNIN-obese rats due to accumulation of intralenticular sorbitol. IUBMB Life, 65(5), 472–478. https://doi.org/10.1002/iub.1163
Reddy, P. Y., Giridharan, N. V., & Reddy, G. B. (2012). Activation of sorbitol pathway in metabolic syndrome and increased susceptibility to cataract in Wistar-obese rats. Molecular Vision, 18, 495–503.
Reddy, S. S., Shruthi, K., Reddy, V. S., Raghu, G., Suryanarayana, P., Giridharan, N. V., & Reddy, G. B. (2014). Altered ubiquitin-proteasome system leads to neuronal cell death in a spontaneous obese rat model. Biochimica Et Biophysica Acta, 1840(9), 2924–2934. https://doi.org/10.1016/j.bbagen.2014.06.005
Strinic, D., Halle, Z. B., Luetic, K., Nedic, A., Petrovic, I., Sucic, M., Posilovic, G. Z., Balenovic, D., Strbe, S., Udovicic, M., Drmic, D., Stupnisek, M., Bencic, M. L., Seiwerth, S., & Sikiric, P. (2017). BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sciences, 186, 66–79. https://doi.org/10.1016/j.lfs.2017.08.006
Potnuri, A. G., Allakonda, L., Appavoo, A., Saheera, S., & Nair, R. R. (2018). Association of histamine with hypertension-induced cardiac remodeling and reduction of hypertrophy with the histamine-2-receptor antagonist famotidine compared with the beta-blocker metoprolol. Hypertension Research: Official Journal of the Japanese Society of Hypertension, 41(12), 1023–1035. https://doi.org/10.1038/s41440-018-0109-2
Sagie, A., Larson, M. G., Goldberg, R. J., Bengtson, J. R., & Levy, D. (1992). An improved method for adjusting the QT interval for heart rate (the Framingham heart study). The American Journal of Cardiology, 70(7), 797–801. https://doi.org/10.1016/0002-9149(92)90562-d
Miller, L. E., Hosick, P. A., Wrieden, J., Hoyt, E., & Quindry, J. C. (2012). Evaluation of arrhythmia scoring systems and exercise-induced cardioprotection. Medicine and Science in Sports and Exercise, 44(3), 435–441. https://doi.org/10.1249/MSS.0b013e3182323f8b
Venu, L., Harishankar, N., Prasanna Krishna, T., & Raghunath, M. (2004). Maternal dietary vitamin restriction increases body fat content but not insulin resistance in WNIN rat offspring up to 6 months of age. Diabetologia, 47(9), 1493–1501. https://doi.org/10.1007/s00125-004-1506-4
Potnuri, A. G., Allakonda, L., Appavoo, A., Saheera, S., & Nair, R. R. (2016). Targeting histamine-2 receptor for prevention of cardiac remodelling in chronic pressure overload. International Journal of Cardiology. https://doi.org/10.1016/j.ijcard.2015.10.040
Yin, F. C., Spurgeon, H. A., Rakusan, K., Weisfeldt, M. L., & Lakatta, E. G. (1982). Use of tibial length to quantify cardiac hypertrophy: Application in the aging rat. The American Journal of Physiology, 243(6), H941-947. https://doi.org/10.1152/ajpheart.1982.243.6.H941
Reddy, G. K., & Enwemeka, C. S. (1996). A simplified method for the analysis of hydroxyproline in biological tissues. Clinical Biochemistry, 29(3), 225–229. https://doi.org/10.1016/0009-9120(96)00003-6
Roden, D. M., Woosley, R. L., & Primm, R. K. (1986). Incidence and clinical features of the quinidine-associated long QT syndrome: Implications for patient care. American Heart Journal, 111(6), 1088–1093. https://doi.org/10.1016/0002-8703(86)90010-4
Glassman, A. H., & Bigger, J. T. (2001). Antipsychotic drugs: Prolonged QTc interval, torsade de pointes, and sudden death. The American Journal of Psychiatry, 158(11), 1774–1782. https://doi.org/10.1176/appi.ajp.158.11.1774
Meyer-Massetti, C., Cheng, C. M., Sharpe, B. A., Meier, C. R., & Guglielmo, B. J. (2010). The FDA extended warning for intravenous haloperidol and torsades de pointes: How should institutions respond? Journal of Hospital Medicine, 5(4), E8-16. https://doi.org/10.1002/jhm.691
Schrijver, E. J., Verstraaten, M., Van De Ven, P. M., Bet, P. M., Van Strien, A. M., De Cock, C., & Nanayakkara, P. W. (2018). Low dose oral haloperidol does not prolong QTc interval in older acutely hospitalised adults: a subanalysis of a randomised double-blind placebo-controlled study. Journal of Geriatric Cardiology, 15(6), 401–407. https://doi.org/10.11909/j.issn.1671-5411.2018.06.003
Lopaschuk, G. D., Folmes Clifford, D. L., & Stanley, W. C. (2007). Cardiac energy metabolism in obesity. Circulation Research, 101(4), 335–347. https://doi.org/10.1161/CIRCRESAHA.107.150417
Rodrigues, J. C., McIntyre, B., Dastidar, A. G., Lyen, S. M., Ratcliffe, L. E., Burchell, A. E., Hart, E. C., Bucciarelli-Ducci, C., Hamilton, M. C., Paton, J. F., Nightingale, A. K., & Manghat, N. E. (2016). The effect of obesity on electrocardiographic detection of hypertensive left ventricular hypertrophy: Recalibration against cardiac magnetic resonance. Journal of Human Hypertension, 30(3), 197–203. https://doi.org/10.1038/jhh.2015.58
Elffers, T. W., de Mutsert, R., Lamb, H. J., Maan, A. C., Macfarlane, P. W., van Dijk, K. W., Rosendaal, F. R., Jukema, J. W., & Trompet, S. (2017). Association of metabolic syndrome and electrocardiographic markers of subclinical cardiovascular disease. Diabetology & Metabolic Syndrome, 9(1), 40. https://doi.org/10.1186/s13098-017-0238-9
Narayanan, K., Zhang, L., Kim, C., Uy-Evanado, A., Teodorescu, C., Reinier, K., Zheng, Z. J., Gunson, K., Jui, J., & Chugh, S. S. (2015). QRS fragmentation and sudden cardiac death in the obese and overweight. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. https://doi.org/10.1161/JAHA.114.001654
Anumonwo, J. M. B., & Herron, T. (2018). Fatty infiltration of the myocardium and arrhythmogenesis: Potential cellular and molecular mechanisms. Frontiers in Physiology. https://doi.org/10.3389/fphys.2018.00002
Cunningham, K. S., Spears, D. A., & Care, M. (2019). Evaluation of cardiac hypertrophy in the setting of sudden cardiac death. Forensic Sciences Research, 4(3), 223–240. https://doi.org/10.1080/20961790.2019.1633761
Artham, S. M., Lavie, C. J., Milani, R. V., & Ventura, H. O. (2009). Obesity and hypertension, heart failure, and coronary heart disease—risk factor, paradox, and recommendations for weight loss. The Ochsner Journal, 9(3), 124–132.
Messerli, F. H., Christie, B., DeCarvalho, J. G. R., Aristimuno, G. G., Suarez, D. H., Dreslinski, G. R., & Frohlich, E. D. (1981). Obesity and essential hypertension: hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Archives of Internal Medicine, 141(1), 81–85. https://doi.org/10.1001/archinte.1981.00340010073016
Aronow, W. S. (2017). Hypertension and left ventricular hypertrophy. Annals of Translational Medicine. https://doi.org/10.21037/atm.2017.06.14
González-Juanatey, J. R., García-Acuña, J. M., Pose, A., Varela, A., Calvo, C., Cabezas-Cerrato, J., & de la Peña, M. G. (1998). Reduction of QT and QTc dispersion during long-term treatment of systemic hypertension with enalapril. The American Journal of Cardiology, 81(2), 170–174. https://doi.org/10.1016/s0002-9149(97)00869-2
de Lucia, C., Eguchi, A., & Koch, W. J. (2018). New insights in cardiac β-adrenergic signaling during heart failure and aging. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2018.00904
Devereux, R. B., & Reichek, N. (1982). Repolarization abnormalities of left ventricular hypertrophy. Clinical, echocardiographic and hemodynamic correlates. Journal of Electrocardiology, 15(1), 47–53. https://doi.org/10.1016/S0022-0736(82)80044-7
Maulik, S. K., & Kumar, S. (2012). Oxidative stress and cardiac hypertrophy: A review. Toxicology Mechanisms and Methods, 22(5), 359–366. https://doi.org/10.3109/15376516.2012.666650
González, J., Valls, N., Brito, R., & Rodrigo, R. (2014). Essential hypertension and oxidative stress: New insights. World Journal of Cardiology, 6(6), 353–366. https://doi.org/10.4330/wjc.v6.i6.353
Baradaran, A., Nasri, H., & Rafieian-Kopaei, M. (2014). Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences, 19(4), 358–367.
Kurokawa, S., Niwano, S., Niwano, H., Murakami, M., Ishikawa, S., Masaki, Y., Tamaki, H., Toda, T., Noda, Y., Shimizu, T., Izumi, T., & Ako, J. (2014). Cardiomyocyte-derived mitochondrial superoxide causes myocardial electrical remodeling by downregulating potassium channels and related molecules. Circulation Journal, 78(8), 1950–1959. https://doi.org/10.1253/circj.CJ-13-1587
Cowling, R. T., Kupsky, D., Kahn, A. M., Daniels, L. B., & Greenberg, B. H. (2019). Mechanisms of cardiac collagen deposition in experimental models and human disease. Translational Research: The Journal of Laboratory and Clinical Medicine, 209, 138–155. https://doi.org/10.1016/j.trsl.2019.03.004
Némethy, G., & Scheraga, H. A. (1986). Stabilization of collagen fibrils by hydroxyproline. Biochemistry, 25(11), 3184–3188. https://doi.org/10.1021/bi00359a016
Sunagawa, T., Shimizu, T., Matsumoto, A., Tagashira, M., Kanda, T., Shirasawa, T., & Nakaya, H. (2014). Cardiac electrophysiological alterations in heart/muscle-specific manganese-superoxide dismutase-deficient mice: Prevention by a dietary antioxidant polyphenol. BioMed Research International. https://doi.org/10.1155/2014/704291
Acknowledgements
The authors would like to acknowledge Indian Council for Medical Research, New Delhi, India for financially funding this work [Grant No: 3/1/3/PDF (18)/2018-HRD]. We thank Director, ICMR—NARFBR, Director, ICMR- NIN, and Director NRIUMSD, Hyderabad for their support and facilities.
Funding
The study is funded by Indian Council for Medical Research, [Grant No: 3/1/3/PDF (18)/2018-HRD].
Author information
Authors and Affiliations
Contributions
Study conception and design: AGP, NHS, PS; Animal breeding husbandry and TOBEC analysis: KPR; Treatment, NIBP, and electrocardiography: AGP, GMH, MHK; Biochemical studies: AGP, KPR, NHS; Statistical analysis: AGP, GMH, MHK, PS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study is approved by Institutional Animal Ethics Committee [Sanction No: ICMR-NIN/IAEC/02/009/2019].
Consent for Publication
Consent for publication is given by all authors.
Additional information
Handling Editor: Vittorio Fineschi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Potnuri, A.G., Reddy, K.P., Suresh, P. et al. Obesity Potentiates the Risk of Drug-Induced Long QT Syndrome - Preliminary Evidence from WNIN/Ob Spontaneously Obese Rat. Cardiovasc Toxicol 21, 848–858 (2021). https://doi.org/10.1007/s12012-021-09675-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09675-w