Abstract
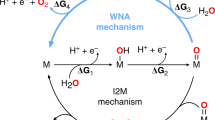
High-throughput computational catalyst studies are typically carried out using density functional theory (DFT) with a single, approximate exchange-correlation functional. In open shell transition metal complexes (TMCs) that are promising for challenging reactions (e.g., C–H activation), the predictive power of DFT has been challenged, and properties are known to be strongly dependent on the admixture of Hartree-Fock (HF) exchange. We carry out a large-scale study of the effect of HF exchange on the predicted catalytic properties of over 1200 mid-row (i.e., Cr, Mn, Fe, Co) 3d TMCs for direct methane-to-methanol conversion. Reaction energy sensitivities across this set depend both on the catalytic rearrangement and ligand chemistry of the catalyst. These differences in sensitivities change both the absolute energetics predicted for a catalyst and its relative performance. Previous observations of the poor performance of global linear free energy relationships (LFERs) hold with both semi-local DFT widely employed in heterogeneous catalysis and hybrid DFT. Narrower metal/oxidation/spin-state specific LFERs perform better and are less sensitive to HF exchange than absolute reaction energetics, except in the case of some intermediate/high-spin states. Importantly, the interplay between spin-state dependent reaction energetics and exchange effects on spin-state ordering means that the choice of DFT functional strongly influences whether the minimum energy pathway is spin-conserved. Despite these caveats, LFERs involving catalysts that can be expected to have closed shell intermediates and low-spin ground states retain significant predictive power.








Similar content being viewed by others
Data Availability
The data sets and codes generated during and/or analyzed during the current study are available in the “methane-to-methanol reaction energy sensitivities” repository, at https://doi.org/10.5281/zenodo.4895418. The codes used in this work are also added to molSimplify.
References
Spivey JJ, Krishna KS, Kumar CSSR, Dooley KM, Flake JC, Haber LH, Xu Y, Janik MJ, Sinnott SB, Cheng YT, Liang T, Sholl DS, Manz TA, Diebold U, Parkinson GS, Bruce DA, de Jongh P (2014) Synthesis, characterization, and computation of catalysts at the center for atomic-level catalyst design. J Phys Chem C 118(35):20043–20069
Sperger T, Sanhueza IA, Kalvet I, Schoenebeck F (2015) Computational studies of synthetically relevant homogeneous organometallic catalysis involving Ni, Pd, Ir, and Rh: an overview of commonly employed DFT methods and mechanistic insights. Chem Rev 115(17):9532–9586
Sperger T, Sanhueza IA, Schoenebeck F (2016) Computation and experiment: a powerful combination to understand and predict reactivities. Acc Chem Res 49(6):1311–1319
Medford AJ, Vojvodic A, Hummelshoj JS, Voss J, Abild-Pedersen F, Studt F, Bligaard T, Nilsson A, Norskov JK (2015) From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J Catal 328:36–42
Cheng GJ, Zhang XH, Chung LW, Xu LP, Wu YD (2015) Computational organic chemistry: bridging theory and experiment in establishing the mechanisms of chemical reactions. J Am Chem Soc 137(5):1706–1725
Vogiatzis KD, Polynski MV, Kirkland JK, Townsend J, Hashemi A, Liu C, Pidko EA (2018) Computational approach to molecular catalysis by 3d transition metals: challenges and opportunities. Chem Rev 119(4):2453–2523
Raugei S, DuBois DL, Rousseau R, Chen S, Ho M-H, Bullock RM, Dupuis M (2015) Toward molecular catalysts by computer. Acc Chem Res 48(2):248–255
Greeley J (2016) Theoretical heterogeneous catalysis: scaling relationships and computational catalyst design. Annu Rev Chem Biomol Eng 7:605–635
Nørskov JK, Bligaard T, Rossmeisl J, Christensen CH (2009) Towards the computational design of solid catalysts. Nat Chem 1(1):37–46
Foscato M, Jensen VR (2020) Automated in silico design of homogeneous catalysts. ACS Catal 10(3):2354–2377
Nandy A, Zhu J, Janet JP, Duan C, Getman RB, Kulik HJ (2019) Machine learning accelerates the discovery of design rules and exceptions in stable metal-oxo intermediate formation. ACS Catal 9:8243–8255
Vogiatzis KD, Haldoupis E, Xiao DJ, Long JR, Siepmann JI, Gagliardi L (2016) Accelerated computational analysis of metal–organic frameworks for oxidation catalysis. J Phys Chem C 120(33):18707–18712. https://doi.org/10.1021/acs.jpcc.6b07115
Kim JY, Kulik HJ (2018) When is ligand pKa a good descriptor for catalyst energetics? In search of optimal CO2 hydration catalysts. J Phys Chem A 122(18):4579–4590
Gani TZH, Kulik HJ (2018) Understanding and breaking scaling relations in single-site catalysis: methane to methanol conversion by FeIV=O. ACS Catal 8:975–986
Cramer CJ, Truhlar DG (2009) Density functional theory for transition metals and transition metal chemistry. Phys Chem Chem Phys 11(46):10757–10816
Janet JP, Zhao Q, Ioannidis EI, Kulik HJ (2017) Density functional theory for modelling large molecular adsorbate-surface interactions: a mini-review and worked example. Mol Simul 43(5–6):327–345
Gaggioli CA, Stoneburner SJ, Cramer CJ, Gagliardi L (2019) Beyond density functional theory: the multiconfigurational approach to model heterogeneous catalysis. ACS Catal 9(9):8481–8502
Jimenez-Hoyos CA, Janesko BG, Scuseria GE (2009) Evaluation of range-separated hybrid and other density functional approaches on test sets relevant for transition metal-based homogeneous catalysts. J Phys Chem A 113(43):11742–11749
Zhao Q, Kulik HJ (2019) Stable surfaces that bind too tightly: can range-separated hybrids or DFT + U improve paradoxical descriptions of surface chemistry? J Phys Chem Lett 10(17):5090–5098
Schimka L, Harl J, Stroppa A, Grüneis A, Marsman M, Mittendorfer F, Kresse G (2010) Accurate surface and adsorption energies from many-body perturbation theory. Nat Mater 9(9):741–744
Kulik HJ (2015) Perspective: treating electron over-delocalization with the DFT plus U method. J Chem Phys 142(24):240901
Yu HS, Li SL, Truhlar DG (2016) Perspective: Kohn-Sham density functional theory descending a staircase. J Chem Phys 145(13):130901
Cohen AJ, Mori-Sanchez P, Yang W (2008) Fractional charge perspective on the band gap in density-functional theory. Phys Rev B 77(11):115123
Perdew JP, Parr RG, Levy M, Balduz JL Jr (1982) Density-functional theory for fractional particle number - derivative discontinuities of the energy. Phys Rev Lett 49(23):1691–1694
Yang WT, Zhang YK, Ayers PW (2000) Degenerate ground states and a fractional number of electrons in density and reduced density matrix functional theory. Phys Rev Lett 84(22):5172–5175
Cohen AJ, Mori-Sanchez P, Yang W (2008) Insights into current limitations of density functional theory. Science 321(5890):792–794
Janesko BG, Proynov E, Kong J, Scalmani G, Frisch MJ (2017) Practical density functionals beyond the overdelocalization–underbinding zero-sum game. J Phys Chem Lett 8(17):4314–4318
Johnson BG, Gonzales CA, Gill PMW, Pople JA (1994) A density-functional study of the simplest hydrogen abstraction reaction - effect of self-interaction correction. Chem Phys Lett 221(1–2):100–108
Ruzsinszky A, Perdew JP, Csonka GI, Vydrov OA, Scuseria GE (2006) Spurious fractional charge on dissociated atoms: pervasive and resilient self-interaction error of common density functionals. J Chem Phys 125(19):194112
Ruzsinszky A, Perdew JP, Csonka GI, Vydrov OA, Scuseria GE (2007) Density functionals that are one- and two- are not always many-electron self-interaction-free, as shown for H-2(+), He-2(+), LiH+, and Ne-2(+). J Chem Phys 126(10):104102
Dutoi AD, Head-Gordon M (2006) Self-interaction error of local density functionals for alkali-halide dissociation. Chem Phys Lett 422(1–3):230–233
Bally T, Sastry GN (1997) Incorrect dissociation behavior of radical ions in density functional calculations. J Phys Chem A 101(43):7923–7925
Zhang Y, Yang W (1998) A challenge for density functionals: self-interaction error increases for systems with a non integer number of electrons. J Chem Phys 109(7):2604–2608
Wilbraham L, Verma P, Truhlar DG, Gagliardi L, Ciofini I (2017) Multiconfiguration pair-density functional theory predicts spin state ordering in iron complexes with the same accuracy as complete active space second-order perturbation theory at a significantly reduced computational cost. J Phys Chem Lett 8(9):2026–2030
Ioannidis EI, Kulik HJ (2017) Ligand-field-dependent behavior of meta-GGA exchange in transition-metal complex spin-state ordering. J Phys Chem A 121(4):874–884
Ioannidis EI, Kulik HJ (2015) Towards quantifying the role of exact exchange in predictions of transition metal complex properties. J Chem Phys 143(3):034104
Mortensen SR, Kepp KP (2015) Spin propensities of octahedral complexes from density functional theory. J Phys Chem A 119(17):4041–4050
Droghetti A, Alfe D, Sanvito S (2012) Assessment of density functional theory for iron(II) molecules across the spin-crossover transition. J Chem Phys 137(12):124303
Ganzenmuller G, Berkaine N, Fouqueau A, Casida ME, Reiher M (2005) Comparison of density functionals for differences between the high-(T-5(2 g)) and low-((1)A(1 g)) spin states of iron(II) compounds. IV. Results for the ferrous complexes [Fe(L)(‘NHS4’)]. J Chem Phys 122:23
Kulik HJ, Cococcioni M, Scherlis DA, Marzari N (2006) Density functional theory in transition-metal chemistry: a self-consistent Hubbard U approach. Phys Rev Lett 97(10):103001
Tozer DJ, De Proft F (2005) Computation of the hardness and the problem of negative electron affinities in density functional theory. J Phys Chem A 109(39):8923–8929
Teale AM, De Proft F, Tozer DJ (2008) Orbital energies and negative electron affinities from density functional theory: insight from the integer discontinuity. J Chem Phys 129(4):044110
Peach MJG, Teale AM, Helgaker T, Tozer DJ (2015) Fractional electron loss in approximate DFT and Hartree-Fock theory. J Chem Theory Comput 11(11):5262–5268
Mori-Sanchez P, Cohen AJ, Yang WT (2008) Localization and delocalization errors in density functional theory and implications for band-gap prediction. Phys Rev Lett 100:14
Mahler A, Janesko BG, Moncho S, Brothers EN (2018) When Hartree-Fock exchange admixture lowers DFT-predicted barrier heights: natural bond orbital analyses and implications for catalysis. J Chem Phys 148(24)
Janet JP, Kulik HJ (2017) Predicting electronic structure properties of transition metal complexes with neural networks. Chem Sci 8:5137–5152. https://doi.org/10.1039/C7SC01247K
Reiher M, Salomon O, Hess BA (2001) Reparameterization of hybrid functionals based on energy differences of states of different multiplicity. Theor Chem Acc 107(1):48–55
Coskun D, Jerome SV, Friesner RA (2016) Evaluation of the performance of the B3LYP, PBE0, and M06 DFT functionals, and DBLOC-corrected versions, in the calculation of redox potentials and spin splittings for transition metal containing systems. J Chem Theory Comput 12(3):1121–1128
Haunschild R, Henderson TM, Jimenez-Hoyos CA, Scuseria GE (2010) Many-electron self-interaction and spin polarization errors in local hybrid density functionals. J Chem Phys 133(13):134116
Mori-Sanchez P, Cohen AJ, Yang WT (2006) Many-electron self-interaction error in approximate density functionals. J Chem Phys 125(20)
Schmidt T, Kummel S (2016) One- and many-electron self-interaction error in local and global hybrid functionals. Phys Rev B 93(16)
Kim MC, Sim E, Burke K (2013) Understanding and reducing errors in density functional calculations. Phys Rev Lett 111:7
Zheng X, Liu M, Johnson ER, Contreras-Garcia J, Yang W (2012) Delocalization error of density-functional approximations: a distinct manifestation in hydrogen molecular chains. J Chem Phys 137(21):214106
Simm GN, Reiher M (2016) Systematic error estimation for chemical reaction energies. J Chem Theory Comput 12(6):2762–2773
Sutton JE, Guo W, Katsoulakis MA, Vlachos DG (2016) Effects of correlated parameters and uncertainty in electronic-structure-based chemical kinetic modelling. Nat Chem 8(4):331–337
Walker E, Ammal SC, Terejanu GA, Heyden A (2016) Uncertainty quantification framework applied to the water–gas shift reaction over Pt-based catalysts. J Phys Chem C 120(19):10328–10339
Wellendorff J, Lundgaard KT, Mogelhoj A, Petzold V, Landis DD, Norskov JK, Bligaard T, Jacobsen KW (2012) Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys Rev B 85(23):235149
Medford AJ, Wellendorff J, Vojvodic A, Studt F, Abild-Pedersen F, Jacobsen KW, Bligaard T, Nørskov JK (2014) Assessing the reliability of calculated catalytic ammonia synthesis rates. Science 345(6193):197–200
Sumaria V, Krishnamurthy D, Viswanathan V (2018) Quantifying confidence in DFT predicted surface Pourbaix diagrams and associated reaction pathways for chlorine evolution. ACS Catal 8(10):9034–9042
Christensen R, Hansen HA, Vegge T (2015) Identifying systematic DFT errors in catalytic reactions. Catal Sci Technol 5(11):4946–4949
Wellendorff J, Silbaugh TL, Garcia-Pintos D, Norskov JK, Bligaard T, Studt F, Campbell CT (2015) A benchmark database for adsorption bond energies to transition metal surfaces and comparison to selected DFT functionals. Surf Sci 640:36–44
Houchins G, Viswanathan V (2017) Quantifying confidence in density functional theory predictions of magnetic ground states. Phys Rev B 96(13):134426
Gani TZH, Kulik HJ (2017) Unifying exchange sensitivity in transition metal spin-state ordering and catalysis through bond valence metrics. J Chem Theory Comput 13:5443–5457
Busch M, Fabrizio A, Luber S, Hutter J, Corminboeuf C (2018) Exploring the limitation of molecular water oxidation catalysts. J Phys Chem C 122(23):12404–12412. https://doi.org/10.1021/acs.jpcc.8b03935
Janesko BG, Scuseria GE (2008) Hartree-Fock orbitals significantly improve the reaction barrier heights predicted by semi local density functionals. J Chem Phys 128(24):244112
Gani TZH, Kulik HJ (2016) Where does the density localize? Convergent behavior for global hybrids, range separation, and DFT + U. J Chem Theory Comput 12:5931–5945
Liu F, Kulik HJ (2020) Impact of approximate DFT density delocalization error on potential energy surfaces in transition metal chemistry. J Chem Theory Comput 16(1):264–277. https://doi.org/10.1021/acs.jctc.9b00842
Oloo W, Que N Jr (2015) Bioinspired nonheme iron catalysts for C–H and C–C bond oxidation: insights into the nature of the metal-based oxidants. Acc Chem Res 48(9):2612–2621. https://doi.org/10.1021/acs.accounts.5b00053
Que L Jr, Tolman WB (2008) Biologically inspired oxidation catalysis. Nature 455(7211):333–340. https://doi.org/10.1038/nature07371
Biswas AN, Puri M, Meier KK, Oloo WN, Rohde GT, Bominaar EL, Munck E, Que L Jr (2015) Modeling TauD-J: a high-spin nonheme oxoiron (IV) complex with high reactivity toward C–H bonds. J Am Chem Soc 137(7):2428–2431. https://doi.org/10.1021/ja511757j
Engelmann X, Monte-Perez I, Ray K (2016) Oxidation reactions with bioinspired mononuclear non-heme metal-oxo complexes. Angew Chem Int Ed 55(27):7632–7649. https://doi.org/10.1002/anie.201600507
Hammond C, Forde MM, Rahim A, Hasbi M, Thetford A, He Q, Jenkins RL, Dimitratos N, Lopez-Sanchez JA, Dummer NF (2012) Direct catalytic conversion of methane to methanol in an aqueous medium by using copper‐promoted Fe‐ZSM‐5. Angew Chem Int Ed 51(21):5129–5133
Jones C, Taube D, Ziatdinov VR, Periana RA, Nielsen RJ, Oxgaard J, Goddard WA (2004) Selective oxidation of methane to methanol catalyzed, with C-H activation, by homogeneous, cationic gold. Angew Chem Int Ed 116(35):4726–4729
Palkovits R, Antonietti M, Kuhn P, Thomas A, Schüth F (2009) Solid catalysts for the selective low-temperature oxidation of methane to methanol. Angew Chem Int Ed 48(37):6909–6912
Hull JF, Balcells D, Sauer EL, Raynaud C, Brudvig GW, Crabtree RH, Eisenstein O (2010) Manganese catalysts for C–H activation: an experimental/theoretical study identifies the stereoelectronic factor that controls the switch between hydroxylation and desaturation pathways. J Am Chem Soc 132(22):7605–7616. https://doi.org/10.1021/ja908744w
Balcells D, Moles P, Blakemore JD, Raynaud C, Brudvig GW, Crabtree RH, Eisenstein O (2009) Molecular recognition in Mn-catalyzed C–H oxidation. Reaction mechanism and origin of selectivity from a DFT perspective. Dalton Trans 30:5989–6000
Latimer AA, Kulkarni AR, Aljama H, Montoya JH, Yoo JS, Tsai C, Abild-Pedersen F, Studt F, Nørskov JK (2017) Understanding trends in C-H bond activation in heterogeneous catalysis. Nat Mater 16(2):225–229. https://doi.org/10.1038/nmat4760
Christensen R, Hansen HA, Dickens CF, Nørskov JK, Vegge T (2016) Functional independent scaling relation for ORR/OER catalysts. J Phys Chem C 120(43):24910–24916. https://doi.org/10.1021/acs.jpcc.6b09141
Fajin JLC, Vines F, Cordeiro MNDS, Illas F, Gomes JRB (2016) Effect of the exchange-correlation potential on the transferability of Bronsted-Evans-Polanyi relationships in heterogeneous catalysis. J Chem Theory Comput 12(5):2121–2126
Curnan MT, Kitchin JR (2015) Investigating the energetic ordering of stable and metastable TiO2 polymorphs using DFT + U and hybrid functionals. J Phys Chem C 119(36):21060–21071
Rosen AS, Notestein JM, Snurr RQ (2019) Structure–activity relationships that identify metal–organic framework catalysts for methane activation. ACS Catal 9(4):3576–3587. https://doi.org/10.1021/acscatal.8b05178
Liao P, Getman RB, Snurr RQ (2017) Optimizing open iron sites in metal – organic frameworks for ethane oxidation: a first-principles study. ACS Appl Mater Interfaces 9(39):33484–33492. https://doi.org/10.1021/acsami.7b02195
Pellizzeri S, Jones IA, Doan HA, Snurr RQ, Getman RB (2016) Using gas-phase clusters to screen porphyrin-supported nanocluster catalysts for ethane oxidation to ethanol. Catal Lett 146(12):2566–2573. https://doi.org/10.1007/s10562-016-1890-7
Wodrich MD, Sawatlon B, Busch M, Corminboeuf C (2021) The genesis of molecular volcano plots. Acc Chem Res 54(5):1107–1117
Anand M, Rohr B, Statt MJ, Nørskov JK (2020) Scaling relationships and volcano plots in homogeneous catalysis. J Phys Chem Lett 11(20):8518–8526
Busch M, Wodrich MD, Corminboeuf C (2015) Linear scaling relationships and volcano plots in homogeneous catalysis–revisiting the Suzuki reaction. Chem Sci 6(12):6754–6761
Andrikopoulos PC, Michel C, Chouzier S, Sautet P (2015) In silico screening of iron-oxo catalysts for CH bond cleavage. ACS Catal 5(4):2490–2499
Nandy A, Kulik HJ (2020) Why conventional design rules for C–H activation fail for open-shell transition-metal catalysts. ACS Catal 10(24):15033–15047. https://doi.org/10.1021/acscatal.0c04300
Szécsényi Á, Khramenkova E, Chernyshov IY, Li G, Gascon J, Pidko EA (2019) Breaking linear scaling relationships with secondary interactions in confined space: a case study of methane oxidation by Fe/ZSM-5 zeolite. ACS Catal 9(10):9276–9284. https://doi.org/10.1021/acscatal.9b01914
Pérez-Ramírez J, López N (2019) Strategies to break linear scaling relationships. Nat Catal 2(11):971–976
Marshall-Roth T, Libretto NJ, Wrobel AT, Anderton KJ, Pegis ML, Ricke ND, Van Voorhis T, Miller JT, Surendranath Y (2020) A pyridinic Fe-N 4 macrocycle models the active sites in Fe/N-doped carbon electrocatalysts. Nat Commun 11(1):1–14
Liu F, Yang T, Yang J, Xu E, Bajaj A, Kulik HJ (2019) Bridging the homogeneous-heterogeneous divide: modeling spin and reactivity in single atom catalysis. Front Chem 7:219
Xu H, Cheng D, Cao D, Zeng XC (2018) A universal principle for a rational design of single-atom electrocatalysts. Nat Catal 1(5):339–348
Sours T, Patel A, Nørskov J, Siahrostami S, Kulkarni A (2020) Circumventing scaling relations in oxygen electrochemistry using metal–organic frameworks. J Phys Chem Lett 11(23):10029–10036
Abram S-L, Monte-Perez I, Pfaff FF, Farquhar ER, Ray K (2014) Evidence of two-state reactivity in alkane hydroxylation by Lewis-acid bound copper-nitrene complexes. Chem Commun 50(69):9852–9854
Zhu B, Guan W, Yan L-K, Su Z-M (2016) Two-state reactivity mechanism of benzene C–C activation by trinuclear titanium hydride. J Am Chem Soc 138(35):11069–11072
Schwarz H (2017) Menage-a-trois: single-atom catalysis, mass spectrometry, and computational chemistry. Catal Sci Technol 7(19):4302–4314
Liu WG, Zhang LL, Liu X, Liu XY, Yang XF, Miao S, Wang WT, Wang AQ, Zhang T (2017) Discriminating catalytically active FeNx species of atomically dispersed Fe-N-C catalyst for selective oxidation of the C–H bond. J Am Chem Soc 139(31):10790–10798
Ricciarelli D, Belpassi L, Harvey JN, Belanzoni P (2020) Spin-forbidden reactivity of transition metal oxo species: exploring the potential energy surfaces. Chem Eur J 26(14):3080–3089. https://doi.org/10.1002/chem.201904314
Harvey JN (2007) Understanding the kinetics of spin-forbidden chemical reactions. Phys Chem Chem Phys 9(3):331–343. https://doi.org/10.1039/b614390c
Harvey JN (2014) Spin-forbidden reactions: computational insight into mechanisms and kinetics. Wiley Interdiscip Rev Comput Mol Sci 4(1):1–14. https://doi.org/10.1002/wcms.1154
Hirao H, Kumar D, Que L Jr, Shaik S (2006) Two-state reactivity in alkane hydroxylation by non-heme iron-oxo complexes. J Am Chem Soc 128(26):8590–8606. https://doi.org/10.1021/ja061609o
Shaik S, Danovich D, Fiedler A, Schroder D, Schwarz H (1995) 2-State reactivity in organometallic gas-phase ion chemistry. Helv Chim Acta 78(6):1393–1407
Schroder D, Shaik S, Schwarz H (2000) Two-state reactivity as a new concept in organometallic chemistry. Acc Chem Res 33(3):139–145
Groves JT, McClusky GA (1976) Aliphatic hydroxylation via oxygen rebound. Oxygen transfer catalyzed by iron. J Am Chem Soc 98(3):859–861. https://doi.org/10.1021/ja00419a049
Ufimtsev IS, Martinez TJ (2009) Quantum chemistry on graphical processing units. 3. Analytical energy gradients, geometry optimization, and first principles molecular dynamics. J Chem Theory Comput 5(10):2619–2628
Ioannidis EI, Gani TZH, Kulik HJ (2016) molSimplify: a toolkit for automating discovery in inorganic chemistry. J Comput Chem 37:2106–2117. https://doi.org/10.1002/jcc.24437
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open babel: an open chemical toolbox. J Cheminf 3:33. https://doi.org/10.1186/1758-2946-3-33
O’Boyle NM, Morley C, Hutchison GR (2008) Pybel: a python wrapper for the open babel cheminformatics toolkit. Chem Cent J 2:5. https://doi.org/10.1186/1752-153X-2-5
Nandy A, Duan C, Janet JP, Gugler S, Kulik HJ (2018) Strategies and software for machine learning accelerated discovery in transition metal chemistry. Ind Eng Chem Res 57(42):13973–13986
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98(45):11623–11627
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104
Becke AD, Johnson ER (2005) A density-functional model of the dispersion interaction. J Chem Phys 123(15):154101
Wadt WR, Hay PJ (1985) Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J Chem Phys 82(1):284–298. https://doi.org/10.1063/1.448800
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82(1):270–283
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods.9. Extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54(2):724–728
Wang L-P, Song C (2016) Geometry optimization made simple with translation and rotation coordinates. J Chem Phys 144(21):214108
Saunders VR, Hillier IH (1973) A “Level-Shifting” method for converging closed shell Hartree-Fock wave functions. Int J Quantum Chem 7(4):699–705. https://doi.org/10.1002/qua.560070407
Nandy A, Chu DBK, Harper DR, Duan C, Arunachalam N, Cytter Y, Kulik HJ (2020) Large-scale comparison of 3d and 4d transition metal complexes illuminates the reduced effect of exchange on second-row spin-state energetics. Phys Chem Chem Phys 22(34):19326–19341. https://doi.org/10.1039/d0cp02977g
Latimer AA, Kakekhani A, Kulkarni AR, Nørskov JK (2018) Direct methane to methanol: the selectivity–conversion limit and design strategies. ACS Catal 8(8):6894–6907. https://doi.org/10.1021/acscatal.8b00220
Bowman DN, Jakubikova E (2012) Low-spin versus high-spin ground state in pseudo-octahedral iron complexes. Inorg Chem 51(11):6011–6019
Kepp KP (2016) Theoretical study of spin crossover in 30 iron complexes. Inorg Chem 55(6):2717–2727
Acknowledgements
The authors acknowledge primary support for the catalyst design screen by the National Science Foundation under Grant numbers CBET-1704266 and CBET-1846426. A.N. was partially supported by a National Science Foundation Graduate Research Fellowship under Grant #1122374. Initial conception and data set generation for this study was supported by the Department of Energy under Grant number DE-SC0012702. Algorithm and workflow development as well as data collection strategies were supported by the Office of Naval Research under Grant numbers N00014-17-1-2956, N00014-18-1-2434, and N00014-20-1-2150. This work was carried out in part using computational resources from the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation Grant number ACI-1548562. H.J.K. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund, an AAAS Marion Milligan Mason Award, and an Alfred P. Sloan Fellowship in Chemistry, which supported this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by VV and AN. The first draft of the manuscript was written by VV, revised by HJK, and all authors commented on and revised versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vennelakanti, V., Nandy, A. & Kulik, H.J. The Effect of Hartree-Fock Exchange on Scaling Relations and Reaction Energetics for C–H Activation Catalysts. Top Catal 65, 296–311 (2022). https://doi.org/10.1007/s11244-021-01482-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01482-5