Abstract
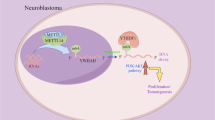
This study aims to investigate the biological role of 6-methyladenine (m6A) methylation in inducing the carcinogenesis of glioma and its proliferation. Relative levels of ALKBH5 and glucose-6-phosphate dehydrogenase (G6PD) in glioma tissues and cell lines were determined by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot. Gain-of-function and loss-of-function approaches were used to investigate the role of ALKBH5 in mediating proliferation and energy metabolism of glioma cells. The regulatory effect of ALKBH5 on G6PD was analyzed using m6A-qRT-PCR. Our results showed that ALKBH5 was upregulated in glioma, which stimulated glioma cells to proliferate. Serving as a m6A eraser, ALKBH5 demethylated the target transcript G6PD and enhanced its mRNA stability, thereby promoting G6PD translation and activating the pentose phosphate pathway (PPP). Collectively, ALKBH5 stimulates glioma cells to proliferate through erasing the m6A methylation of G6PD, which can be utilized as a potential therapeutic target for glioma.




Similar content being viewed by others
References
Goodenberger ML, Jenkins RB (2012) Genetics of adult glioma. Cancer Genet 205:613–621
Thakkar JP, Prabhu VC, Peters KB, Lukas RV (2021) What is new in neuro-oncology? Neurol Clin 39:163–179
Bagherian A, Mardani R, Roudi B, Taghizadeh M, Banfshe HR, Ghaderi A, Davoodvandi A, Shamollaghamsari S, Hamblin MR, Mirzaei H (2020) Combination therapy with nanomicellar-curcumin and temozolomide for in vitro therapy of glioblastoma multiforme via wnt signaling pathways. J Mol Neurosci 70:1471–1483
Dadgostar E, Fallah M, Izadfar F, Heidari-Soureshjani R, Aschner M, Tamtaji OR, Mirzaei H (2021) Therapeutic potential of resveratrol in the treatment of glioma: insights into its regulatory mechanisms. Mini Rev Med Chem. https://doi.org/10.2174/1389557521666210406164758
Ghaemmaghami AB, Mahjoubin-Tehran M, Movahedpour A, Morshedi K, Sheida A, Taghavi SP, Mirzaei H, Hamblin MR (2020) Role of exosomes in malignant glioma: microRNAs and proteins in pathogenesis and diagnosis. Cell Commun Signal 18:120
Shabaninejad Z, Pourhanifeh MH, Movahedpour A, Mottaghi R, Nickdasti A, Mortezapour E, Shafiee A, Hajighadimi S, Moradizarmehri S, Sadeghian M, Mousavi SM, Mirzaei H (2020) Therapeutic potentials of curcumin in the treatment of glioblstoma. Eur J Med Chem 188:112040
Khani P, Nasri F, Khani CF, Saeidi F, Sadri NJ, Tabibkhooei A, Mirzaei H (2019) Genetic and epigenetic contribution to astrocytic gliomas pathogenesis. J Neurochem 148:188–203
Hu J, Xiao Q, Dong M, Guo D, Wu X, Wang B (2020) Glioblastoma immunotherapy targeting the innate immune checkpoint CD47-SIRPalpha axis. Front Immunol 11:593219
Jiang P, Du W, Wu M (2014) Regulation of the pentose phosphate pathway in cancer. Protein Cell 5:592–602
Kowalik MA, Columbano A, Perra A (2017) Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Front Oncol 7:87
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12:931–947
Zhang C, Zhang Z, Zhu Y, Qin S (2014) Glucose-6-phosphate dehydrogenase: a biomarker and potential therapeutic target for cancer. Anticancer Agents Med Chem 14:280–289
Yang CA, Huang HY, Lin CL, Chang JG (2018) G6PD as a predictive marker for glioma risk, prognosis and chemosensitivity. J Neurooncol 139:661–670
Globisch D, Pearson D, Hienzsch A, Bruckl T, Wagner M, Thoma I, Thumbs P, Reiter V, Kneuttinger AC, Muller M, Sieber SA, Carell T (2011) Systems-based analysis of modified tRNA bases. Angew Chem Int Ed Engl 50:9739–9742
He C (2010) Grand challenge commentary: RNA epigenetics? Nat Chem Biol 6:863–865
Desrosiers R, Friderici K, Rottman F (1974) Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA 71:3971–3975
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG, Riggs AD, He C, Shi Y (2017) m(6)A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep 18:2622–2634
Mochizuki S, Okada Y (2007) ADAMs in cancer cell proliferation and progression. Cancer Sci 98:621–628
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bogler O, Majumder S, He C, Huang S (2017) m(6)A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 31:591–606
Xuan JJ, Sun WJ, Lin PH, Zhou KR, Liu S, Zheng LL, Qu LH, Yang JH (2018) RMBase v2.0: deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res 46:D327–D334
Chandola U, Das R, Panda B (2015) Role of the N6-methyladenosine RNA mark in gene regulation and its implications on development and disease. Brief Funct Genomics 14:169–179
Heyn H, Esteller M (2015) An adenine code for DNA: a second life for N6-methyladenine. Cell 161:710–713
Loos RJ, Yeo GS (2014) The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol 10:51–61
Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, Lu Z, Bosmans RP, Dai Q, Hao YJ, Yang X, Zhao WM, Tong WM, Wang XJ, Bogdan F, Furu K, Fu Y, Jia G, Zhao X, Liu J, Krokan HE, Klungland A, Yang YG, He C (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49:18–29
Thalhammer A, Bencokova Z, Poole R, Loenarz C, Adam J, O’Flaherty L, Schodel J, Mole D, Giaslakiotis K, Schofield CJ, Hammond EM, Ratcliffe PJ, Pollard PJ (2011) Human AlkB homologue 5 is a nuclear 2-oxoglutarate dependent oxygenase and a direct target of hypoxia-inducible factor 1alpha (HIF-1alpha). PLoS ONE 6:e16210
Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, He X, Semenza GL (2016) Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci USA 113:E2047–E2056
Mathupala SP, Rempel A, Pedersen PL (1997) Aberrant glycolytic metabolism of cancer cells: a remarkable coordination of genetic, transcriptional, post-translational, and mutational events that lead to a critical role for type II hexokinase. J Bioenerg Biomembr 29:339–343
Wood T (1986) Physiological functions of the pentose phosphate pathway. Cell Biochem Funct 4:241–247
Chen H, Yue JX, Yang SH, Ding H, Zhao RW, Zhang S (2009) Overexpression of transketolase-like gene 1 is associated with cell proliferation in uterine cervix cancer. J Exp Clin Cancer Res 28:43
Yang HC, Wu YH, Yen WC, Liu HY, Hwang TL, Stern A, Chiu DT (2019) The redox role of G6PD in cell growth, cell death, and cancer. Cells 8:1055
Kuo W, Lin J, Tang TK (2000) Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3 cells and induces tumors in nude mice. Int J Cancer 85:857–864
Batetta B, Bonatesta RR, Sanna F, Putzolu M, Mulas MF, Collu M, Dessi S (2002) Cell growth and cholesterol metabolism in human glucose-6-phosphate dehydrogenase deficient lymphomononuclear cells. Cell Prolif 35:143–154
Li D, Zhu Y, Tang Q, Lu H, Li H, Yang Y, Li Z, Tong S (2009) A new G6PD knockdown tumor-cell line with reduced proliferation and increased susceptibility to oxidative stress. Cancer Biother Radiopharm 24:81–90
Tian WN, Braunstein LD, Pang J, Stuhlmeier KM, Xi QC, Tian X, Stanton RC (1998) Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem 273:10609–10617
Kekec Y, Paydas S, Tuli A, Zorludemir S, Sakman G, Seydaoglu G (2009) Antioxidant enzyme levels in cases with gastrointesinal cancer. Eur J Intern Med 20:403–406
Wang J, Duan Z, Nugent Z, Zou JX, Borowsky AD, Zhang Y, Tepper CG, Li JJ, Fiehn O, Xu J, Kung HJ, Murphy LC, Chen HW (2016) Reprogramming metabolism by histone methyltransferase NSD2 drives endocrine resistance via coordinated activation of pentose phosphate pathway enzymes. Cancer Lett 378:69–79
Tekade RK, Sun X (2017) The Warburg effect and glucose-derived cancer theranostics. Drug Discov Today 22:1637–1653
Acknowledgements
This study was supported by Scientific Research Fund Project of Hebei Provincial Health Commission (20211353).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Chen, Y., Wang, L. et al. ALKBH5 Promotes the Proliferation of Glioma Cells via Enhancing the mRNA Stability of G6PD. Neurochem Res 46, 3003–3011 (2021). https://doi.org/10.1007/s11064-021-03408-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03408-9