Abstract
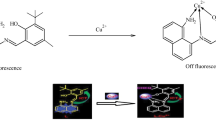
A novel fluorimetric and colorimetric chemosensor (1O) was synthesized with diarylethene-rhodamine unit and characterized by ESI–MS, 1H NMR, and 13C NMR. The chemosensor can selectively recognize extremely low concentrations of Hg2+ over a variety of metal ions with remarkable colorimetric and fluorescent responses. The colorimetric and fluorescent changes were ascribed the reaction between 1O and Hg2+ destructed the rhodamine hydrazide into open-ring form which was proved by mass spectrometry and nuclear magnetic titration analyses. The detection limits of the UV absorption and fluorescence methods for Hg2+ were found to be 0.708 μM and 24.6 nM, respectively. Moreover, the chemosensor exhibited excellent photochromism and outstanding fatigue resistance property under alternating UV and visible light irradiation. The application potential of the chemosensor was demonstrated with the qualitative detection of Hg2+ in real water samples.









Similar content being viewed by others

Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ren MG, Deng BB, Wang JY, Liu ZR, Lin WY (2015) A dual-emission fluorescence-enhanced probe for imaging copper (II) ions in lysosomes. J Mater Chem B 3:6746–6752. https://doi.org/10.1039/c5tb01184a
Wang CC, Liu YQ, Cheng JY, Song JH, Zhao YF, Ye Y (2015) Efficient FRET-based fuorescent ratiometric chemosensors for Fe3+ and its application in living cells. Journal of Luminescence 157:143–148. https://doi.org/10.1016/j.jlumin.2014.08.039
Patnaik R, Padhy RN (2018) Comparative study on toxicity of methylmercury chloride and methylmercury hydroxide to the human neuroblastoma cell line SH-SY5Y. Environmental Science and Pollution Research International 25:20606–20614. https://doi.org/10.1007/s11356-018-2164-2
de Freitas AS, Funck VR, Rotta Mdos S, Bohrer D, Morschbacher V, Puntel RL, Nogueira CW, Farina M, Aschner M, Rocha JBT (2009) Diphenyl diselenide a simple organoselenium compound, decrease methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Research Bulletin 79:77–84. https://doi.org/10.1016/j.brainresbull.2008.11.001
Dan W, Deng XK, Yu YH (2019) A novel turn-on fluorescent probe for Hg2+ detection based on rhodamine B spirolactam derivative. International Journal of Environmental Analytical Chemistry 99:1515–1527. https://doi.org/10.1080/03067319.2019.1625339
Mahato P, Saha S, Suresh E, Di Liddo R, Parnigotto PP, Conconi MT, Kesharwani MK, Ganguly B, Das A (2012) Ratiometric detection of Cr3+ and Hg2+ by a Naphthalimide-rhodamine based fluorescent probe. Inorganic Chemistry 51:1769–1777. https://doi.org/10.1021/ic202073q
Kraithong S, Sirirak J, Soisuwan K, Wanichacheva N, Swanglap P (2018) Enhancing sensitivity of novel Hg2+ fluorescent sensor via plasmonic enhancement of silver nanoparticles. Sensors and Actuators B: Chemical 258:694–703. https://doi.org/10.1016/j.snb.2017.11.049
Tang X, Wang Y, Han J, Ni L, Zhang HQ, Li C, Li J, Qiu Y (2018) A novel fluorescent probe based on biphenyl and rhodamine for multi-metal ion recognition and its application. Dalton Transactions 47:3378–3387. https://doi.org/10.1039/c7dt04629d
Rani BK, John SA (2018) Fluorogenic mercury ion sensor based on pyrene-amino mercapto thiadiazole unit. Journal of Hazardous Materials 343:98–106. https://doi.org/10.1016/j.jhazmat.2017.09.028
Chen SX, Zhang SS, A RH, Han YF, (2020) A new rhodamine probe with large stokes shift for Hg2+ detection and its application in real sample analysis. Tetrahedron Letters 61(27):152077. https://doi.org/10.1016/j.tetlet.2020.152077
Yuan ZH, Yang YS, Lv PC, Zhu HL (2020) Recent progress in small-molecule fluorescent probes for detecting mercury ions. Crit Rev Anal Chem. https://doi.org/10.1080/10408347.2020.1797466
Petdum A, Panchan W, Sirirak J, Promarak V, Sooksimuang T, Wanichacheva N (2018) Colorimetric and fluorescent sensing of a new FRET system via [5] helicene and rhodamine 6G for Hg2+ detection. New J Chem 42:1396–1402. https://doi.org/10.1039/c7nj04129b
Lee YH, Park N, Park YB, Hwang YJ, Kang C, Kim JS (2014) Organelle-selective fluorescent Cu2+ ion probes: revealing the endoplasmic reticulum as a reservoir for Cu-overloading. Chemical Communications 50:3197–3200. https://doi.org/10.1039/c4cc00091a
Wu XF, Ma QJ, Wei XJ, Hou YM, Zhu X (2013) A selective fluorescent sensor for Hg2+ based on covalently immobilized naphthalimide derivative. Sensors and Actuators B: Chemical 183:565–573. https://doi.org/10.1016/j.snb.2013.04.024
Song F, Yang C, Shao XT, Du L, Zhu J, Kan C (2019) A reversible “turn-off-on” fluorescent probe for real-time visualization of mercury(II) in environmental samples and its biological applications. Dyes Pigm 165:444–450. https://doi.org/10.1016/j.dyepig.2019.02.054
Wang QM, Jin L, Wang WL, Hu TX, Chen C (2019) Rhodamine derivatives as selective “naked-eye” colorimetric and fluorescence off-on sensor for Hg2+ in aqueous solution and its applications in bioimaging. J Lumin 209:411–419. https://doi.org/10.1016/j.jlumin.2019.02.024
Zhang XF, Wang TR, Cao XQ, Shen SL (2020) A near-infrared rhodamine-based lysosomal pH probe and its application in lysosomal pH rise during heat shock. Spectrochim Acta Part A Mol Biomol Spectrosc 227:117761 https://doi.org/10.1016/j.saa.2019.117761
Das S, Rissanen K, Sahoo P (2019) Rare crystal structure of open spirolactam ring along with the closed-ring form of a rhodamine derivative: Sensing of Cu2+ Ions from Spinach. American Chemical Society Omega, 4:5270–5274. https://doi.org/10.1021/acsomega.9b00053
Bao XF, Shi JX, Nie XM, Zhou BJ, Wang XL, Zhang LY, Liao H, Pang T (2014) A new Rhodamine B-based “on-off” chemical sensor with high selectivity and sensitivity toward Fe3+ and its imaging in living cells. Bioorganic and Medicinal Chemistry 22:4826–4835. https://doi.org/10.1016/j.bmc.2014.06.054
Kan C, Song F, Shao XT, Wu LY, Zhu J (2020) Fe (III) induced fluorescent probe based on triamine and rhodamine derivatives and its applications in biological imaging. J Photochem Photobiol A: Chem 390:112306. https://doi.org/10.1016/j.jphotochem.2019.112306
Huang K, Yue YX, Jiao XJ, Liu C, Wang Q, He S, Zhao LC, Zeng XS (2017) Fluorescence regulation of 4-aminobenzofluoran and its applications for Cu2+-selective fluorescent probe and bioimaging. Dyes Pigm 143:379–386. https://doi.org/10.1016/j.dyepig.2017.04.064
Qiao B, Sun SG, Jiang N, Zhang S, Peng XJ (2014) A ratiometric fluorescent probe for determining Pd2+ ions based on coordination. Dalton Transactions 43:4626–4630. https://doi.org/10.1039/c3dt53541j
Aich K, Goswami S, Das S, Mukhopadhyay CD, Quah CK, Fun HK (2015) Cd2+ triggered the FRET “ON”: A new molecular switch for the ratiometric detection of Cd2+ with live-cell imaging and bound X-ray structure. Inorg Chem 54:7309–7315. https://doi.org/10.1021/acs.inorgchem.5b00784
Tang JL, Li CY, Li YF, Lu X, Qi HR (2015) A highly sensitive and selective fluorescent probe for trivalent aluminum ion based on rhodamine derivative in living cells. Anal Chim Acta 888:155–161. https://doi.org/10.1016/j.aca.2015.07.033
Wu GW, Wang ZY, Zhang WX, Chen W, Jin XX, Lu HF (2019) A novel rhodamine B and purine derivative-based fluorescent chemosensor for detection of palladium. Inorganic Chemistry Communications 102:233–239. https://doi.org/10.1016/j.inoche.2019.02.038
Li G, Liu G, Zhang DB, Pu SZ (2016) A new fluorescence probe based on fluorescein-diarylethene fluorescence resonance energy transfer system for rapid detection of Cd2+. Tetrahedron 72:6390–6396. https://doi.org/10.1016/j.tet.2016.08.037
Wu ZY, Xu ZY, Tan HY, Li X, Yan JW, Dong CZ, Zhang L (2019) Two novel rhodamine-based fluorescent probes for the rapid and sensitive detection of Fe3+: Experimental and DFT calculations. Spectrochimica Acta Part A: Molecular Biomolecular Spectroscopy 213:167–175. https://doi.org/10.1016/j.saa.2019.01.032
Shree GJ, Sivaraman G, Siva A, Chellappa D (2019) Anthracene- and pyrene-bearing imidazoles as turn-on fluorescent chemosensor for aluminum ion in living cells. Dyes and Pigments 163:204–212. https://doi.org/10.1016/j.dyepig.2018.11.061
Tang TG, Guo WB, Zhang YP, Xu DM (2019) A novel 1, 8-naphthalimide-based "turn-on" fluorescent sensor for Fe3+. J Fluoresc 29:445–450. https://doi.org/10.1007/s10895-019-02354-8
Cao XR, Zhang FF, Bai YJ, Ding XH, Sun W (2019) A highly selective “turn-on” fluorescent probe for detection of Fe3+ in cells. J Fluoresc 29:425–434. https://doi.org/10.1007/s10895-019-02351-x
Song F, Yang C, Liu HB, Gao ZG, Zhu J, Bao XF, Kan C (2019) Dual-binding pyridine and rhodamine B conjugate derivatives as fluorescent chemosensors for ferric ions in aqueous media and living cells. Analyst 144:3094–3102. https://doi.org/10.1039/c8an01915k
Bozkurt E, Gul HI (2018) A novel pyrazoline-based fluorometric “Turn-off” sensing for Hg2+. Sensor Actuat B-Chem 255:814–825. https://doi.org/10.1016/j.snb.2017.08.062
Wang Y, Ding H, Wang S, Fan C, Tu Y, Liu G, Pu S (2019) A ratiometric and colorimetric probe for detecting Hg2+ based on naphthalimide–rhodamine and its staining function in cell imaging. RSC Advances 9:11664–11669. https://doi.org/10.1039/c9ra01459d
Manoj K, Naresh K, Vandana B (2011) Naphthalimide appended rhodamine derivative: Through bond energy transfer for sensing of Hg2+ ions. Org Lett 13(6):1422–1425. https://doi.org/10.1021/ol2001073
Orriach-Fernández FJ, Medina-Castillo A, Fernández-Sánchez JF et al (2013) Hg2+-selective sensing film based on the incorporation of a rhodamine 6G derivative into a novel hydrophilic water-insoluble copolymer. Anal Methods 5:6642–6648. https://doi.org/10.1039/c3ay40717a
Chereddy NR, Saranraj K, Barui AK, Patra CR, Rao VJ, Thennarasu S (2014) Donor atom selective coordination of Fe3+ and Cr3+ trigger fluorophore specific emission in a rhodamine-naphthalimide dyad. RSC Advances 4:24324–24327. https://doi.org/10.1039/c4ra02797c
Goswami S, Das S, Aich K, Sarkar D, Mondal TK, Quah CK, Fun HK (2013) CHEF induced highly selective and sensitive turn-on fluorogenic and colorimetric sensor for Fe3+. Dalton Transactions 42:15113–15119. https://doi.org/10.1039/c3dt51974k
Huang L, Hou FP, Cheng J, Xi PX, Chen FJ, Bai DC, Zeng ZZ (2012) Selective off-on fluorescent chemosensor for detection of Fe3+ ions in aqueous media. Organic and Biomolecular Chemistry 10:9634–9638. https://doi.org/10.1039/c2ob26258d
Sunnapu O, Kotla NG, Maddiboyina B, Singaravadivel S, Sivaraman G (2016) A rhodamine based “turn-on” fluorescent probe for Pb (II) and live cell imaging. RSC Advances 6:656–660. https://doi.org/10.1039/c5ra20482h
Zhou LY, Wang QQ, Zhang XB, Tan WH (2015) Through-bond energy transfer-based ratiometric two-photon probe for fluorescent imaging of Pd2+ ions in living cells and tissues. Analytical Chemistry 87:4503–4507. https://doi.org/10.1021/acs.analchem.5b00505
Wang M, Liu XM, Lu HZ, Wang HM, Qin ZH (2015) Highly selective and reversible chemosensor for Pd2+ detected by fluorescence, colorimetry, and test paper. ACS Applied Materials and Interfaces 7:1284–1289. https://doi.org/10.1021/am507479m
Zeng RF, Lan JS, Wu T, Liu L, Liu Y, Ho RJY, Ding Y, Zhang T (2020) A novel mitochondria-targetted near-infrared fluorescent probe for selective and colorimetric detection of sulfite and its application in vitro and vivo. Food Chemistry 318:126358. https://doi.org/10.1016/j.foodchem.2020.126358
Li M, Jiang XJ, Wu HH, Lu HL, Li HY, Xu H, Zang SQ, Mak TC (2015) A dual functional probe for “turn-on” fluorescence response of Pb2+ and colorimetric detection of Cu2+ based on a rhodamine derivative in aqueous media. Dalton Transactions 44:17326–17334. https://doi.org/10.1039/c5dt02731d
Zhao H, Ding HC, Kang HM, Fan CB, Liu G, Pu SZ (2019) A solvent-dependent chemosensor for fluorimetric detection of Hg2+ and colorimetric detection of Cu2+ based on a new diarylethene with a rhodamine B unit. RSC Advances 9:42155–42162. https://doi.org/10.1039/c9ra08557b
Wang S, Ding HC, Wang YS, Fan CB, Liu G, Pu SZ (2019) Novel multi-responsive fluorescence switch for Hg2+ and UV/visible lights based on diarylethene-rhodamine derivative. Tetrahedron 75:1517–1524. https://doi.org/10.1016/j.tet.2019.01.071
Xu HT, Ding HC, Li G, Fan CB, Liu G, Pu SZ (2017) A highly selective fluorescent chemosensor for Fe3+ based on a new diarylethene with a rhodamine 6G unit. RSC Advances 7:29827–29834. https://doi.org/10.1039/c7ra04728b
Sun Y, Dong B, Lu Y, Song W, Mehmood AH, Lin W (2020) A sensitive and selective fluorescent probe for the detection of endogenous peroxynitrite (ONOO-) in living cells. Analytical Methods 12:2841–2845. https://doi.org/10.1039/d0ay00012d
Wang L, Ren MG, Li ZH, Dai LX, Lin WY (2019) Development of a FRET-based ratiometric fluorescent probe to monitor the changes in palladium(ii) in aqueous solution and living cells. New Journal of Chemistry 43:552–555. https://doi.org/10.1039/c8nj04866e
Guo SL, Liu G, Fan CB, Pu SZ (2018) A new diarylethene-derived probe for colorimetric sensing of Cu(II) and fluorometric sensing of Cu(II) and Zn(II): Photochromism and High Selectivity. Sensor Act B-Chem. 266:603–613. https://doi.org/10.1016/j.snb.2018.03.168
Shi F, Cui SQ, Liu HL, Pu SZ (2020) A high selective fluorescent sensor for Cu2+ in solution and test paper strips. Dyes and Pigments 173:107914. https://doi.org/10.1016/j.dyepig.2019.107914
Qiu SY, Lu MM, Cui SQ, Wang Z, Pu SZ (2019) A bifunctional sensor based on diarylethene for the colorimetric recognition of Cu2+ and fluorescence detection of Cd2+. RSC Advances 9:29141–29148. https://doi.org/10.1039/c9ra04965g
Wang Z, Cui SQ, Qiu SY, Pu SZ (2018) A highly selective fluorescence “turn-on” sensor for Ca2+ based on diarylethene with a triazozoyl hydrazine unit. RSC Advances 8:29295–29300. https://doi.org/10.1039/c8ra06039h
Pu SZ, Tong ZP, Liu G, Wang RJ (2013) Multi-addressable molecular switches based on a new diarylethene salicylal Schiff base derivative. Journal of Materials Chemistry C 1(31):4726–4739. https://doi.org/10.1039/c3tc30804a
Liu YM, Shen R, Ru JX, Yao X, Yang Y, Liu HL, Tang XL, Bai DC, Zhang GL, Liu WS (2016) A reversible rhodamine 6G-based fluorescence turn-on probe for Fe3+ in water and its application in living cell imaging. RSC Advances 6:111754–111759. https://doi.org/10.1039/c5ra09758d
Zhang YR, Zhao ZM, Su L, Miao JY, Zhao BX (2016) A ratiometric fluorescence sensor for HOCl based on a FRET platform and application in living cells. RSC Advances 6:17059–17063. https://doi.org/10.1039/c5ra26027b
Jiao XY, Xiao YS, Li Y, Liang MW, Xie XL, Wang X, Tang B (2018) Evaluating drug-induced liver injury and its remission via discrimination and imaging of HClO- and H2S with a two-photon fluorescent probe. Anal Chem 90:7510–7516. https://doi.org/10.1021/acs.analchem.8b01106
Adhikari S, Ta S, Ghosh A, Guria S, Pal A, Ahir M, Adhikary A, Hira SK, Manna PP, Das D (2019) A 1,8 Naphthalimide anchor rhodamine B based FRET probe for ratiometric detection of Cr3+ ion in living cells. Journal of Photochemistry and Photobiology A: Chemistry 372:49–58. https://doi.org/10.1016/j.jphotochem.2018.12.010
Biswal B, Bag B (2013) Preferences of rhodamine coupled (aminoalkyl)-piperazine probes towards Hg (II) ion and their FRET mediated signaling. Organic and Biomolecular Chemistry 11:4975–4992. https://doi.org/10.1039/c3ob40648b
Li YJ, Pan WH, Zheng CH, Pu SZ (2020) A diarylethene derived Fe3+ fluorescent chemosensor and its application in wastewater analysis. Journal of Photochemistry and Photobiology A: Chemistry 389:112282. https://doi.org/10.1016/j.jphotochem.2019.112282
Acknowledgements
The author was very appreciation for the support of the National Natural Science Foundation of China (41867052, 21662015, 21861017, 1867053), the Science Funds of the Education Office of Jiangxi, China (GJJ190613), and the Masters' Innovative Foundation of Jiangxi Science and Technology Normal University (YC2019-X09).
Funding
This work was supported by the National Natural Science Foundation of China (Grant numbers [41867052, 21662015, 21861017, 1867053]), the Science Funds of the Education Office of Jiangxi, China (Grant numbers [GJJ190613], and the Masters' Innovative Foundation of Jiangxi Science and Technology Normal University (Grant numbers [YC2019-X09]).
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by Xiumei Li, Heng Zhao and Huimin Kang; the first draft of the manuscript was written by Xiumei Li and revised by Xue Li and Congbin Fan; Gang Liu and Shouzhi Pu gave guidance ideas and experimental platform.
Corresponding authors
Ethics declarations
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The participant has consented to the submission of this work to the journal.
Conflicts of Interest
The authors have no conflicts of interest to declare are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Li, X., Zhao, H. et al. A Novel Diarylethene-rhodamine Unit Based Chemosensor for Fluorimetric and Colorimetric Detection of Hg2+. J Fluoresc 31, 1513–1523 (2021). https://doi.org/10.1007/s10895-021-02775-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02775-4