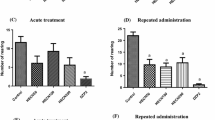
Experiments on rats in a depressive-like state model caused by reserpine employed the elevated plus-maze and open-field tests and showed that 9-(4-ethylpiperazino-1-carbonyl)fluoren-9-ol hydrochloride, a structural analog of the M-choline blocker amizil, at a dose of 10 mg/kg (1/16 LD50) in an intraperitoneal administration course (30 d) had an anxiolytic effect, stimulated exploratory activity, and normalized the vegetative reactions of experimental animals. The strength of the anxiolytic effect of this compound was comparable to that of the benzodiazepine anxiolytic diazepam. Statistically significant differences in the numbers of visits to the open and closed arms and the times spent in those arms in the elevated-plus maze test after administration of the test compound or diazepam were not found. The obtained results indicate that the structural analog of amizil is promising for development as a drug producing an anxiolytic effect in neurotic states to eliminate emotional instability.
Similar content being viewed by others
References
G. L. Vyshkovskii (chief ed.), Russian Drug Registry (RLS). Encyclopedia of Drugs [in Russian], 15th Ed., Moscow (2007), pp. 1156 – 1159.
M. D. Mashkovskii, Drugs [in Russian], 16th Ed., Umerenkov: Novaya Volna, Moscow (2014).
E. Castrenand and T. Rantamaki, Dev. Neurobiol., 70(5), 289 – 297 (2010).
P. J. Fitzgerald, Med. Hypotheses, 80(6), 823 – 826 (2013).
A. A. Crawford, S. Lewis, D. Nutt, et al., Psychopharmacology (Berlin, Ger.), 231(15), 2921 – 2931 (2014).
N. A. Losev, N. S. Sapronov, L. K. Khnychenko, and P. D. Shabanov, Pharmacology of New Cholinergic Agents (Pharmacology – Clinic) [in Russian], Art-ekspress, St. Petersburg (2015).
O. Hromatka, O. Kraupp, and L. Stentzel, “The syntheses of new acylpiperazines [in German],” Monatsh. Chem., 85, 1215 – 1222 (1954).
Federal Law “On the protection of animals from cruelty,” MH RF Order No. 267 of Jun. 19, 2003.
A. N. Mironov and N. D. Bunatyan, Handbook for Preclinical Drug Trials [in Russian], Part 1 – 2, Grif i K, Moscow (2012).
S. E. File, J. Pharm. Pharmacol., 36, 837 – 840 (1984).
Ya. Buresh, O. Bureshova, and J. P. Houston, Methods and Basic Experiments in Studies of the Brain and Behavior, Vysshaya Shkola, Moscow (1991), pp. 119 – 122.
V. P. Fisenko (ed.), Handbook for Experimental (Preclinical) Study of New Drugs [in Russian], ZAO IIA Remedium, Moscow (2000), pp. 131 – 137.
L. K. Khnychenko, V. E. Gmiro, et al., RU Pat. No. 2,700,576, Sept. 18, 2019; Byull., No. 26 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 55, No. 4, pp. 25 – 28, April, 2021.
Rights and permissions
About this article
Cite this article
Khnychenko, L.K., Yakovleva, E.E., Gmiro, V.E. et al. Synthesis and Anxiolytic Activity of 9-(4-Ethylpiperazino-1-Carbonyl)Fluoren-9-OL Hydrochloride, A Structural Analog of the M-Choline Blocker Amizil. Pharm Chem J 55, 336–339 (2021). https://doi.org/10.1007/s11094-021-02423-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02423-y