Abstract
This work presented the studies with the natural zeolite—clinoptilolite as the catalyst for the isomerization of geraniol. During the research, it turned out that the studied process is much more complicated, and not only isomerization takes place in it, but also dehydration, oxidation, dimerization, cyclization and fragmentation of the carbon chain. Geraniol is an organic raw material which can be obtained not only by a chemical synthesis but also from plants (renewable biomass) by distillation or extraction method, for example a source of geraniol can be a plant—geranium. Before catalytic tests clinoptilolite was characterized by the instrumental methods, such as: XRD, porosity studies—nitrogen adsorption at 77 K, SEM, EDXRF, and FT-IR. Gas chromatography analyses showed that the main products of geraniol isomerization process were 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol. The selectivity of 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol depended on the temperature, catalyst content and reaction time. These parameters were changed in the following ranges: 80–150 °C (temperature), 5–15 wt% (catalyst content) and 15–1440 min. (reaction time). The most favorable conditions for 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol obtaining were: temperature 140 ºC, catalyst content 12.5 wt% and reaction time 180 min. At these conditions, the conversion of geraniol amounted to 98 mol%, and the selectivities of 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol amounted to 14 and 47 mol%, respectively.
Similar content being viewed by others
Introduction
Natural zeolites are hydrated aluminosilicates with skeletal structure. Their structure is made up of numerous pores and channels, with a large external surface, which makes them among others catalytic active porous materials. The most common natural zeolites are: clinoptilolite, stilbite, chabasite, mordenite, analcym, and laumitite. Their chemical composition varies, depending on the rocks in which they occur [1]. Zeolites (natural and synthetic) have been drawing the attention of researchers studying catalytic reactions in organic chemistry for many years. This is because the defined, repeatable structure of these materials creates the possibility of conducting chemical reactions directed at only one product—the shape-selective properties of zeolites. Thanks to this property, zeolites have been used, among others, in oxidation, epoxidation and hydroxylation reactions. In addition, zeolites, due to their heterogeneity, are a material that is easily separated from the post-reaction mixture, regenerated and recycled to the process. Currently, research is being conducted on increasing the durability of their structures, the inhibiting the metals leaching, because metals the active centers of these materials, and on methods of their regeneration. Natural zeolites are also of great interest due to their availability and price, and simple methods to prepare them for the role of a catalyst for chemical reactions. For zeolites, it is often sufficient to calcine, dry or wash them with an acid solution of the appropriate concentration to increase of their activity.
Clinoptilolite has a microporous skeletal structure of AlO4 tetrahedrons and SiO4. Aluminum and silicon are here interconnected via oxygen atoms. The crystal lattice of clinoptilolite has open voids in the form cages and tubules. These cages can adsorb various substances. Under natural conditions, water is mainly adsorbed, which can then be removed from the pores by heating, at what the structure of zeolite does not change [2] (Fig. 1).
The three-dimensional structure of clinoptilolite affects its properties, among others: negative crystal lattice charges, easy exchange of non-network cations, uniform size of micropores, thermal and hydrothermal stability. The above-mentioned properties allow the use of clinoptilolite in a wide range of processes such as ion exchange, catalysis, crude oil processing, purification, and gas separation. In addition, clinoptilolite is used in medicine, cosmetology, architecture, and in environmental protection [3].
The state of the art regarding the chemical transformations of geraniol at the conditions of isomerization process performing is not widely described in the literature. Yu et al. [4] described the process of isomerization of geraniol catalyzed by FeCl2⋅6H2O. Linalool and α-terpineol were obtained as products. The process was carried out using acetonitrile as solvent, into which geraniol and a catalyst were introduced. The reaction time was 4 h, while the synthesis was carried out at the room temperature.
Haese et al. [5] described the process of isomerization of geraniol, in both pure and a mixture with nerol. It turned out that the results obtained for pure geraniol are the same as for the mixture with nerol.
Srivastava et al. [6] described the isomerization of geraniol by gamma radiation. The geraniol-methanol solution was irradiated, which in turn caused isomerization to nerol and linalool. The maximum conversion of geraniol obtained in the process was 30%.
Ramishvili et al. [7] and Tsitsishvili et al. [8] described the isomerization of geraniol using BEA micro- and mesoporous zeolites. The process was carried out at the temperature range of 27 to 150 °C, for 1–2 h, in the liquid phase (without any solvent), under an inert gas atmosphere (nitrogen, and argon). Studies show that geraniol reacted mainly to linalool and nerol, as well as to (2E, 6E)-6,11-dimethyl-2,6,10-dodecatrien-1-ol (farnesol). Introduction of mesopores into the microporous structure caused an increase in the acidity of the catalysts, and thus an increase in the conversion of geraniol to 99% and increase in the tendency to transformation of formed farnesol into geranylgeraniol was observed. By combining the first and last carbon atom in the geranylgeraniol molecule, the macrocyclic thumbergol (C20H34O) was formed, which was then dehydrated to form tunbergen (C20H32). Geraniol conversion reached 40%.
Fajdek-Bieda et al. [9] described the process of isomerization of geraniol using clay mineral—sepiolite as the catalyst. The research included the influence of such parameters as: temperature, catalyst content and reaction time. These parameters changed in the following range: temperature 80–150 ºC, catalyst content 5–15 wt%, and reaction time 15–1440 min. As a result of the process, products such as: β-pinene, ocimenes, linalool, nerol, citrals, izocembrol and thunbergol were obtained. However, the highest selectivity (19 mol%) was obtained for linalool at 100 mol% conversion of geraniol.
Terpenes, including geraniol ((2E)-3,7-dimethylocta-2,6-dien-1-ol, C10H18O, CAS Number 106-24-1), are natural products that can be easily transformed into new and valuable compounds used in industrial production of fragrances, perfumes, aromas or therapeutic agents [10]. It can be products of isomerization, dehydration, oxidation, dimerization, cyclization and fragmentation of the carbon chain. The products of dehydration are β-pinene and ocimenes, the isomerization products are linalool and nerol, the oxidation products are geranial and neral, and the products of dimerization, fragmentation of the carbon chain or cyclization are 6,11-dimethyl-2,6,10-dodecatrien-1-ol ((2E,6E)-6,11-dimethyl-2,6,10-dodecatrien-1-ol, C14H24O) and thumbergol.
β-Pinene (6,6-dimethyl-2-methylidenebicyclo[3.1.1]heptane, C10H16, CAS Number 127-91-3) is the organic chemical compound from the group of bicyclic monoterpenes. The main component of turpentine obtained from pine resin. It is used in cosmetics as an ingredient in perfumes and as a raw material for obtaining other fragrances, e.g. α-terpineol, limonene or bergaptol. In addition, it found use as an anti-inflammatory, expectorant, bronchodilator, and in traditional Chinese medicine as an anti-cancer drug [6].
Ocimene isomers (3,7-dimethylocta-1,3,6-triene, C10H16; α-ocimene CAS Number 6874-44-8, β-ocimene CAS Number 13877-91-3 and allo-ocimene CAS Number 673-84-7) are organic chemical compounds from the monoterpenes group. They occur naturally in essential oils, including in lavender oil and calendula oil. They are used in the perfume industry, and have anti-fungal properties [11].
Linalool (3,7-dimethylocta-1,6-dien-3-ol, C10H18O, CAS Number 78-70-6) is an unsaturated aliphatic alcohol, belonging to the terpenes group, with an intense lily of the valley smell. It is a mixture of two stereoisomers: likareol and coriandrol. It is obtained from essential oils: linal, coriander, orange and others, or synthetically. It is produced by various families, among others: jasmine (basil, mint), laurel (laurel, cinnamon) and rutaceous (citrus), as well as silver birch. It is widely used in the perfume industry as a fragrance or in the form of linaloolyl acetate. Also used as an insecticide. Linalool offers enormous potential in the treatment of leukemia and cervical cancer, and provides new starting points for future cancer research [12]. Neroli oil is widely used in the treatment of acute and chronic inflammation, reducing pain sensitivity and symptoms of menopause, as well as an aromatherapeutic agent to reduce stress and improve the endocrine system. The active compounds contained in this oil have an anticonvulsant effect (treatment of epileptic seizures) and microbiological activity toward bacteria, fungi and yeast, leading to application as an auxiliary in the treatment of bacterial skin infections. Neroli oil is used in cosmetics to promote regeneration of skin cells, improve elasticity, help maintain serum levels and reduce the appearance of wrinkles, stretch marks and scars. Its soothing and calming properties have led to use in the treatment of stress-induced dermatological diseases [13,14,15,16].
Citral ((2E)-3,7-dimethylocta-2,6-dienal, C10H16O, cis-citral CAS Number 5392-40-5 and trans-citral CAS Number 5392-40-5) is the organic chemical compound from the group of aldehydes, a product of oxidation of geraniol or nerol from the group of non-ring terpenes. This compound has an intense lemon-like scent. It has two geometric isomers—E (or trans), known as "citral a" (or geranial) and Z (also cis) or "citral b", also called neral. In nature, it occurs as a mixture of isomers. Under the influence of sulfuric acid, a mixture of cyclic alcohols is formed from it. Citral is used as a fragrance in the perfume industry and as a flavor enhancer in the food industry. It also has antibacterial properties and has been used in the treatment of cancer [17].
Thumbergol (also known as isocembrol,) ((1R,2E,4R,7E,11E)-4-Hydroxycembra-2,7,11-triene, C20H34O, CAS Number 25269-17-4) is a monocyclic diterpene alcohol that has been used in the treatment of cancer and may also have neuroprotective properties [18, 19].
The aim of this work was to study the catalytic activity of clinoptilolite. We chose this natural zeolite for research because of its easy availability and price, and because of our earlier research with this porous material on the isomerization of alpha-pinene and limonene, and it turned out to be very promising. This natural zeolite underwent instrumental examinations (by the method of XRD, SEM, FTIR, and XRF), allowing to obtain its full characteristic of physio-chemical properties. The work presented the studies on the influence of the following parameters on the chemical transformation of geraniol (in the original version, our goal was to study the process of isomerization of geraniol, but during the research it turned out that under the conditions in which the isomerization process was carried out, it also underwent other chemical transformations): temperature, catalyst content and reaction time. Syntheses were carried out at atmospheric pressure, in an air atmosphere and without the use of any solvent, which is advantageous considering the possibility of reaction of the solvent with the geraniol transformation products as well as the necessity to recover and recycle the solvent to the process—which may be of little benefit in view of the cost of such operations. The main functions which was taken into account during choosing the best conditions for geraniol transformations were selectivities of 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol.
Experimental section
Raw materials
Clinoptilolite catalyst was purchased from Sepifeed (Turkey) and geraniol (99 wt%) was purchased from Acros Organics (United States). In addition, the following chromatographic standards (templates) were used during quantitative analyzes by gas chromatography mass spectrometry (GC–MS): citronellol (95%, Sigma Aldrich), citral (95%, Sigma Aldrich), ocimene (90%, Sigma Aldrich), beta-pinene (95%, Fluka), linallol (97%, Acros organics), farnesol (96% Acros organics), nerol (97%, Acros organics), myrcene (technical grade, Sigma Aldrich), geranylgeraniol (85%, Sigma Aldrich), and geraniol (99%, Acros Organics).
Characteristics of clinoptilolite catalyst
X-ray diffraction (XRD) was performed using Empyrean X-ray diffractometer with a Cu Kα radiation source (Malvern Panalytical, UK). Samples were analyzed in the range 5°–30° with step size of 0.02°. The specific surface area (SSA), total pore volume (TPV) and micropore volume (MV) were measured by nitrogen adsorption at 77 K using a QUADRASORB evoTM Gas Sorption Surface Area and Pore Size Analyzer (Quantachrome Instruments, USA). Prior to analysis, clinoptilolite samples were degassed at 250 °C for 20 h under N2. Scanning electron microscopy (SEM) was performed with an ultra-high resolution field emission SEM (Hitachi UHR FE-SEM SU8020, Tokyo, Japan) equipped with the secondary electron detector. Elemental analysis was performed using energy dispersive X-ray fluorescence (EDXRF) spectrometer Epsilon3 (Malvern Panalytical, UK). The structure of clinoptilolite was characterized by UV–Vis (SPECORD M40) analysis in the wavelength range 200–600 nm. In addition, FT-IR spectra were made (Thermo Nicolet 380 apparatus) in the range of wavenumbers from 400 to 4000 cm−1.
Characteristics of clinoptilolite catalyst
X-ray difraction (XRD) was performed using Empyrean X-ray difractometer with a Cu Kα radiation source (Malvern Panalytical, UK). Samples were analyzed in the range 5°–30° with step size of 0.02°. The specific surface area (SSA), total pore volume (TPV) and micropore volume (MV) were measured by nitrogen adsorption at 77 K using a QUADRASORB evoTM Gas Sorption Surface Area and Pore Size Analyzer (Quantachrome Instruments, USA). Prior to analysis, sepiolite samples were degassed at 250 °C for 20 h under N2. Scanning electron microscopy (SEM) was performed with an ultra-high resolution field emission SEM (Hitachi UHR FE-SEM SU8020, Tokyo, Japan) equipped with the secondary electron detector. Elemental analysis was performed using energy dispersive X-ray fluorescence (EDXRF) spectrometer Epsilon3 (Malvern Panalytical, UK). The structure of sepiolite was characterized by UV–Vis (SPECORD M40) analysis in the wavelength range 200–600 nm. In addition, FT-IR spectra were made (Thermo Nicolet 380 apparatus) in the range of wavenumbers from 400 to 4000 cm−1.
Method of isomerization of geraniol and analyses of the post-reaction mixtures
Studies on the course of geraniol isomerization process in the presence of clinoptilolite and determination of the most beneficial conditions of this process were carried out in a glass reactor with the capacity of 25 cm3, equipped with a reflux condenser and a magnetic stirrer with a heating function. The studied parameters were changed in the following ranges: temperature 80–150 ºC, catalyst content 5–15 wt%, and reaction time from 15 min to 24 h.
For qualitative and quantitative analyses, the post-reaction mixture was centrifuged and dissolved in acetone in a 1:3 weight ratio. The qualitative analyses were performed by the GC–MS method using a ThermoQuest apparatus equipped with a Voyager detector and a DB-5 column (filled with phenylmethylsiloxanes, 30 m × 0.25 mm × 0.5 mm). The parameters of analyses were as follows: helium flow of 1 ml/min, the temperature of the sample chamber 200 ºC, the temperature of the detector 250 ºC, the temperature of the furnace—isothermally for 2.5 min at 50 ºC, then increase at the rate of 10 ºC/min to 300 ºC. Quantitative analyses were performed with a Thermo Electron FOCUS chromatograph equipped with a FID detector and a TR-FAME column (filled with cyanopropylphenyl, 30 m × 0.25 mm × 0.25 mm). The parameters of the analyses were as follows: helium flow of 0.7 ml/min, sample chamber temperature of 200 ºC, detector temperature of 250ºC, temperature of the furnace—isothermally for 7 min at 60 ºC then increase at the rate of 15 ºC/min to 240 ºC. In order to determine the composition of the post-reaction mixtures the method of an external standard was used. For establishing the amounts of products and of the unreacted substrate, calibration curves were made for eight measurement points within a concentration range of 0–33 wt%. The FID was maintained at 250 °C. Two methods were used to quantitatively determine the reaction products in post-reaction mixtures: (i) an external standard method using commercially available chemical standards and (ii) an internal standardization method. In the external standard method, 8-point calibration curves for each compound were made within a concentration range of 0–33 wt%.
The mass balances for the syntheses allowed the conversion of geraniol and the selectivities of the different products to be calculated. The method employed for calculating these main functions of the process is as follows:
Discussion
The characteristic of clinoptilolite by instrumental methods
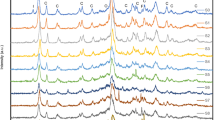
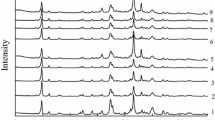
Fig. 2 shows the XRD patterns corresponding to the clinoptilolite. The highest peaks at 14.92, 13.21, 20.41, 23.16 characteristic for clinoptilolite according to JCPDS card 25-1349 were clearly seen at the Fig. 2. but also lower peaks were present. All peaks listed in JCPDS card 25-1349 are denoted by “C”.
The N2 adsorption–desorption isotherm exhibited type II/IV isotherms with hysteresis loops type H3 without adsorption limit at relative pressures close to 1. H3 hysteresis indicates slit-shaped mesopores. The surface area was equal to 38 m2/g. Total pore volume for the pores lower than 180 nm was equal to 0.118 cm3/g. Micropores volume calculated on the basis of t-plot was lower than 0.01 cm3/g. Such low value can be neglected and can be concluded that all the pores were mesopores.
SEM images show (Fig. 3) that clinoptilolite is a plate-shaped material. Its finer grains form larger aggregates. The cleavage of zeolite grains is typical for clinoptilolite and is also a consequence of filtration of hydrothermal solutions. The heterogeneous nature of clinoptilolite grains is shown in Fig. 3. The results of elemental analysis are listed in Table 1.
The FTIR results show (Fig. 4) the water adsorption, as evidenced by the bands within 3500 and 1640 cm−1. The bands at 3444 cm−1 are responsible for the presence of OH groups, while the 1637 cm−1 band refers to the water molecules associated with Na and Ca, present in the zeolite structure. The 1067 cm−1 band is responsible for asymmetric stretching vibrations inside the T-O bonds in TO4 tetrahedrons (T=Si and Al). Bands 796 and 469 cm−1 are assigned to stretching vibrations of O–T–O groups and bending tensions of T–O bonds. These results are similar as those obtained by other authors [20, 21].
Summing up, it can be said that the results of the XRD method confirmed that the tested material is characterized by diffraction peaks characteristic of clinoptilolite, while the porosity tests show that, taking into account its specific surface area and pore size, it may be a porous material with potential use as a catalyst in organic reactions. Additionally, the analysis of clinoptilolite composition shows that Al is present in its composition, which is an active center on which geraniol can be transformed into more useful products.
The influence of temperature of geraniol isomerization
In the first stage, studies on the influence of temperature on the isomerization of geraniol were carried out. The influence of the temperature was studied in the range of 80–150 °C. The initial parameters of the isomerization of geraniol were as follows: catalyst content 10 wt% and the reaction time 3 h. Fig. 5. shows the influence of temperature on the conversion of geraniol and selectivities of appropriate products (beta-pinene, ocimenes, linalool, nerol, cis- and trans citral, 6,11-dimethyl-2,6,10-dodecatrien-1-ol, and thumbergol). It is visible that the conversion of geraniol rises from 3.3 to 97.8 mol% in the range of studied temperatures. But the highest increase of this function is observed in the range of temperatures 120–150 °C. It results also from Fig. 5 that for the temperatures 80–120 °C the only products the studied process were 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol (thumbergol was formed usually with a little higher selectivity than 6,11-dimethyl-2,6,10-dodecatrien-1-ol). In the studied range of temperatures the selectivity of 6,11-dimethyl-2,6,10-dodecatrien-1-ol raises from 0 mol% (temperature of 80 °C) to 27 mol% (temperature of 140 °C) and next decreases to 9 mol% (temperature of 150 °C). For thumbergol it is visible increase in its selectivity from 0 mol% (temperature of 80 °C) to 49 mol% (temperature of 150 °C). For higher temperatures, we can observed formation also other products (lighter products). At the temperature of 130 °C from these lighter products only formation of cis-and trans-citral is observed (selectivity 5.7 and 10.0 mol%, respectively). At the temperature of 140 °C and 150 °C all light products were formed (beta-pinene, ocimenes, linalool, nerol, cis- and trans-citral) but with the highest selectivities were formed ocimenes and trans-citral.
The influence of temperature on the conversion of geraniol and selectivity of the appropriate products over clinoptilolite as the catalyst (we used the glass reactor with the capacity of 25 cm3, equipped with the reflux condenser and the magnetic stirrer with the heating function, first we weighed the right amount of geraniol, and then added the right amount of catalyst, catalyst content amounted 10 wt% and reaction time was 3 h)
Based on the results of the first stage of our studies (the influence of temperature) for the tested process, the reaction scheme presented in Fig. 6. can be proposed.
Moreover, the total selectivity of other products that were not separately determined by the GC method decreased as the temperature decreased from 100 mol% (80 °C) to 8 mol% (140 °C). At lower temperatures, these products include probably myrcene, limonene, trans-farnesol, and trans, trans, trans-geranylgeraniol. However, at higher temperatures, other products are the products originated from consecutive reactions, mainly oligomeric compounds.
We decided that the basis for choosing the most favorable temperature for running the geraniol isomerization process would be the highest value of such function describing this process as the selectivity of transformation to 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol. Thus, we took the temperature of 140 °C as the most beneficial.
The influence of catalyst content of geraniol isomerization
The influence of catalyst content on the course of the isomerization of geraniol over clinoptilolite as the catalyst was studied in the next stage of our catalytic test (Fig. 7). The parameters at which the isomerization was performed were as follows: temperature 140 °C and the reaction time 3 h. Fig. 7. shows that the increase in clinoptilolite content from 2.5 to 12.5 wt% causes the significant increase in the value of the conversion of geraniol (from 29 mol% to 98–100 mol%). But the highest increase was observed in the range of catalyst content from 10 to 15 wt%. As in the case of the first studied parameter (temperature), 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol are the dominant products. The selectivity of the first of them decreases with the increase of the catalyst content from 55 mol% (2.5 wt%) to 15 mol% (15 wt%). In contrast, thumbergol selectivity increases with the increasing in clinoptilolite content from 1 mol% (2.5 wt%) to 44 mol% (15 wt%). Other products with lower molecular weights, such as beta-pinene, ocymenes, linalool, nerol, citrals, were formed during this process with low selectivities. And the highest selectivity from these products achieved ocymenes, linalool and trans-citral.
The influence of clinoptilolite content on the conversion of geraniol and selectivity of the appropriate products over clinoptilolite as the catalyst (we used the glass reactor with the capacity of 25 cm3, equipped with the reflux condenser and the magnetic stirrer with the heating function, first we weighed the right amount of geraniol, and then added the right amount of catalyst, temperature 140 °C and reaction time 3 h)
At this stage of the research, the content of the catalyst amounted to 12.5 wt% was assumed to be the most favorable catalyst content.
The influence of reaction time of geraniol isomerization
The influence of the reaction time on the course of isomerization was examined in the range of 15 min to 24 h. Other parameters (temperature and reaction time) corresponded to the values previously established as the most beneficial. Fig. 8. shows that extending the reaction time to above 3 h increases the value of the conversion of geraniol to 100 mol%. For short reaction times (below 3 h) only are formed: 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol (the selectivity of 6,11-dimethyl-2,6,10-dodecatrien-1-ol decrease from 37 to 29 mol%, and thumbergol from 62 to 47 mol%), while after 3 h not only 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol are formed but also products of isomerization, oxidation and dehydration are detected in the post-reaction mixture but the values of their selectivity (the same as 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol) are relatively low. Above the reaction time of 3 h the selectivity of 6,11-dimethyl-2,6,10-dodecatrien-1-ol decreases from 12 mol% (reaction time 4 h) to 5 mol% (reaction time 24 h). The selectivity of thumbergol also decreases in the range of reaction time from 4 to 24 h from 17 to 9 mol%.
The influence of the reaction time on the conversion of geraniol and selectivity of the appropriate products over sepiolite as the catalyst (we used the glass reactor with the capacity of 25 cm3, equipped with the reflux condenser and the magnetic stirrer with the heating function, first we weighed the right amount of geraniol, and then added the right amount of catalyst, temperature 140 °C and catalyst content 12.5 wt%)
The selectivity of transformation to other organic compounds (not determined by the GC method) increases with prolongation the reaction time to about 83 mol% (reaction time 24 h). This is probably cause by increase in the amount of the oligomerization products in the post-reaction mixture (a sign of this may be the increasing darkening of the post-reaction mixture with the longer isomerization time).
At this stage of the research, the reaction time of 3 h was assumed to be the most favorable reaction time.
Conclusions
The research showed that all parameters (temperature, catalyst content and reaction time) have the significant influence on the course of the conversion of geraniol as well as the selectivities of appropriate products. From the syntheses carried out, it can be observed that the best results of the isomerization were obtained at the temperature of 140 °C, with the catalyst content of 12.5 wt% and during 3 h. The above reaction conditions make it possible to obtain reaction products, mainly 6,11-dimethyl-2,6,10-dodecatrien-1-ol and thumbergol with the highest selectivity possible (14 mol% and 47 mol%, respectively), while at the same time high conversion of geraniol (98 mol%). The use of higher temperature, higher catalyst content and longer reaction time would result in the formation of other products, less desirable, which would constitute only impurities (oxidation products and polymeric compounds), and which would definitely affect the quality of the process itself.
The proposed method of transforming geraniol, characterized by the absence of a solvent in the reaction mixture, is advantageous. The introduction of the solvent to the reaction mixture would cause the solvent to react with geraniol and its transformation products, which would increase the product quantity of this process even more and consequently make the identification, separation and purification of the end products (especially thumbergol) more difficult. It can be considered that the final selectivity of thumbergol is sufficient (47 mol%) with geraniol conversion of 98 mol%. Taking into account the valuable applications of thumbergol in medicine (it is used in the treatment of cancer and may also have neuroprotective properties), the proposed method may be the effective method of obtaining this compound. The use of a solvent in the process under study would also require the use of additional steps of recovery, regeneration and recycling of this solvent to the process. These steps would increase the cost of running this process.
The data availability statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Masters AF, Maschmeyer T (2011) Zeolites—from curiosity to cornerstone. Microporous Mesoporous Mater 142:423–438. https://doi.org/10.1016/j.micromeso.2010.12.026
Armbruster T (1993) Dehydration mechanism of clinoptilolite and heulandite: single-crystal X-ray study of Na-poor, Ca-, K-, Mg-rich clinoptilolite at 100 K. Am Miner 78:260–264
Pleśniak J, Trzop W (2016) Codzienność z zeolitami Analit 2:146–151
Yu W, Wen M, Yang L et al (2002) Ferric chloride catalyzed isomerization and cyclization of geraniol, linalool and nerol. Chin Chem Lett 13:495–496
Haese F, Ebel K, Burkart K, Unverricht S (2006) Method for isomerizing allyl alcohols
Srivastava P, Wagh RS, Naik DG (2010) γ-irradiation: a simple route for isomerization of geraniol into nerol and linalool. Radiochemistry 52:561–564. https://doi.org/10.1134/S1066362210050206
Tsitsishvili V, Ivanova I, Ramishvili T et al (2017) Catalytic conversion of linalool on micro-mesoporous BEA-type zeolite. Bull Georg Natl Acad Sci 11:80–87
Tsitsishvili V, Ramishvili T, Ivanova I et al (2018) Formation of long-chain and macrocyclic compounds during catalytic conversion of geraniol on micro-and micro-mesoporous BEA-type zeolite. Bull Georg Natl Acad Sci 12:62–69
Fajdek A, Agnieszka B, Piotr W, Alicja M (2020) Influence of technological parameters on the isomerization of geraniol using sepiolite. Catal Lett 150:901–911. https://doi.org/10.1007/s10562-019-02987-1
Bieda A, Wróblewska A, Miądlicki P (2019) Healing properties of geraniol—a review of the literature. Pomeranian J Life Sci 65:24–28. https://doi.org/10.21164/pomjlifesci.529
Russo EB (2011) Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 163:1344–1364. https://doi.org/10.1111/j.1476-5381.2011.01238.x
Nissen L, Zatta A, Stefanini I et al (2010) Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 81:413–419. https://doi.org/10.1016/j.fitote.2009.11.010
Laird K, Kurzbach E, Score J et al (2014) Reduction of Legionella spp. in water and in soil by a citrus plant extract vapor. Appl Environ Microbiol 80:6031–6036. https://doi.org/10.1128/AEM.01275-14
Min D, Chen Y, Cai Z, et al (2015) Progress on polymerization of -pinene. In: Proceedings of the 3rd international conference on material, mechanical and manufacturing engineering. Atlantis Press, Paris, France
Roberts WJ, Day AR (1950) A study of the polymerization of α- and β-pinene with friedel—crafts type catalysts. J Am Chem Soc 72:1226–1230. https://doi.org/10.1021/ja01159a044
Zviely M, Li M (2013) Ocimene a versatile floral ingredient. Perfum Flavorist 38:42–45
Zdrojewicz Z, Minczakowska K, Klepacki K (2014) Rola aromaterapii w medycynie. Fam Med Prim Care Rev 16:387–391
Lis-Balchin M (1997) Essential oils and “aromatherapy”: their modern role in healing. J R Soc Health 117:324–329. https://doi.org/10.1177/146642409711700511
Moein M, Zarshenas MM, Delnavaz S (2014) Chemical composition analysis of rose water samples from Iran. Pharm Biol 52:1358–1361. https://doi.org/10.3109/13880209.2014.885062
Olad A, Naseri B (2010) Preparation, characterization and anticorrosive properties of a novel polyaniline/clinoptilolite nanocomposite. Prog Org Coat 67:233–238. https://doi.org/10.1016/j.porgcoat.2009.12.003
Perraki T, Orfanoudaki A (2004) Mineralogical study of zeolites from Pentalofos area, Thrace, Greece. Appl Clay Sci 25:9–16. https://doi.org/10.1016/S0169-1317(03)00156-X
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fajdek-Bieda, A., Wróblewska, A., Miądlicki, P. et al. Clinoptilolite as a natural, active zeolite catalyst for the chemical transformations of geraniol. Reac Kinet Mech Cat 133, 997–1011 (2021). https://doi.org/10.1007/s11144-021-02027-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02027-3